Method for preparing lithium vanadium phosphoric acid of anode material of lithium ion battery under high pressure
A lithium-ion battery, lithium vanadium phosphate technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of slow reaction rate, high reaction temperature, large volume change, etc., to avoid pretreatment steps, reaction The effect of temperature reduction and simple and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Will Li 2 CO 3 , V 2 o 5 and NH 4 h 2 PO 4 Weigh 200g according to the stoichiometric ratio of Li:V:P=3:2:3, mix evenly, and ball mill on a planetary ball mill for 7h; then pretreat at 300°C for 5h under air atmosphere, and after natural cooling, the powder is obtained Shape product; Add low molecular weight phenolic resin 50g as carbon source in above-mentioned powdery product, ball mill 12h again in planetary ball mill, in N 2 In gas atmosphere, sintering is carried out at 900° C. for 12 h under 1 MPa pressure to obtain carbon-coated lithium vanadium phosphate positive electrode material.
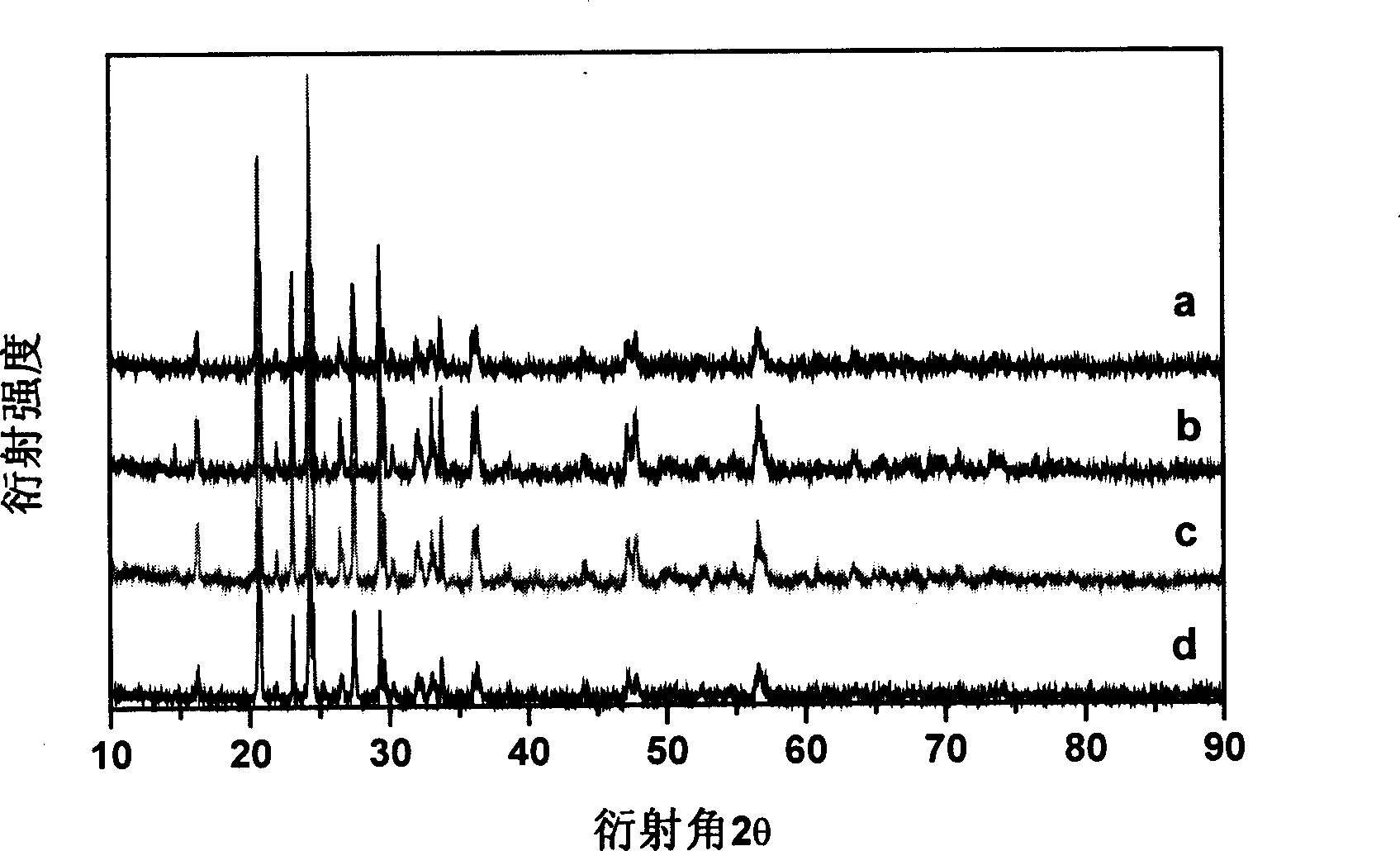
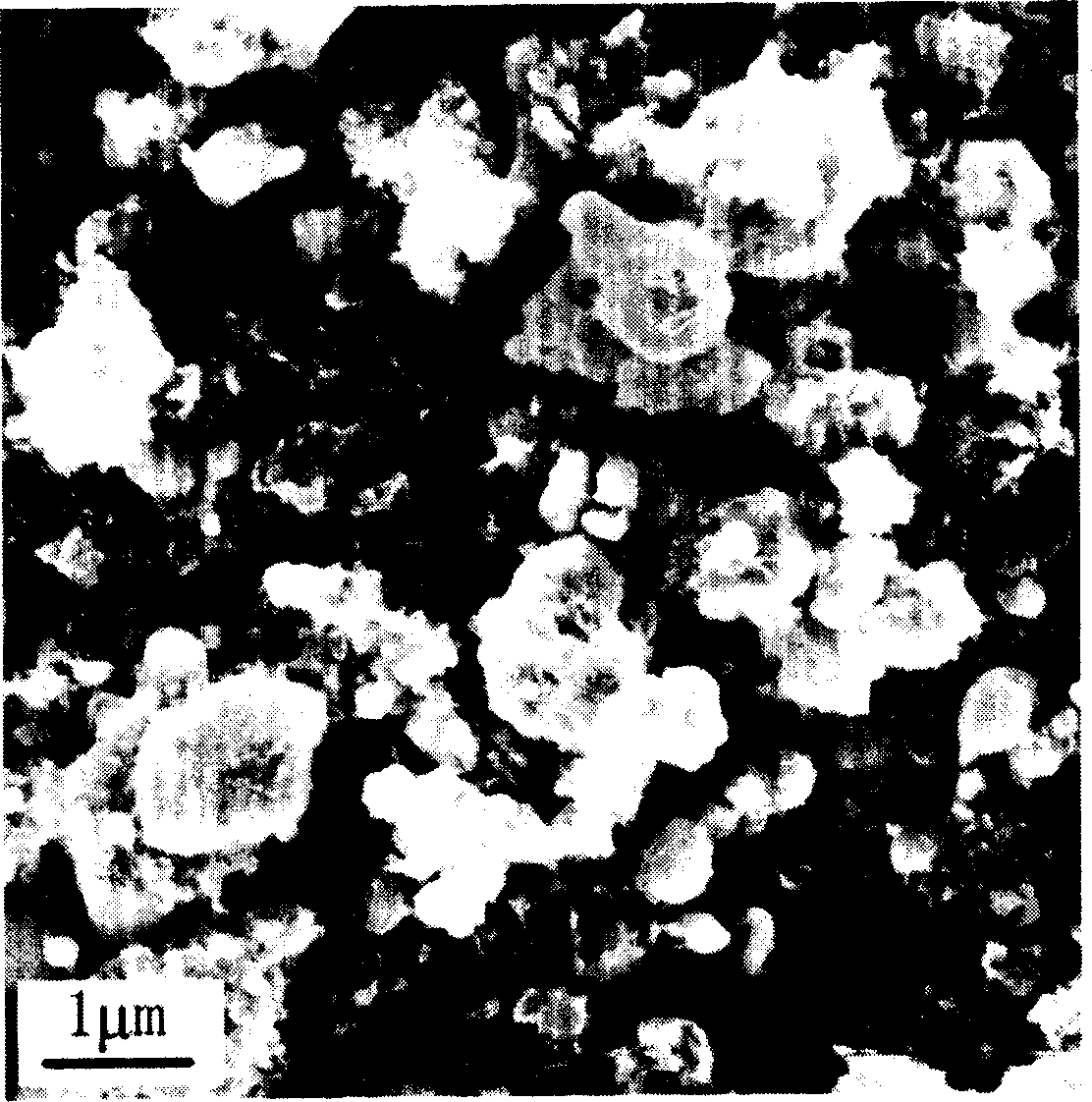
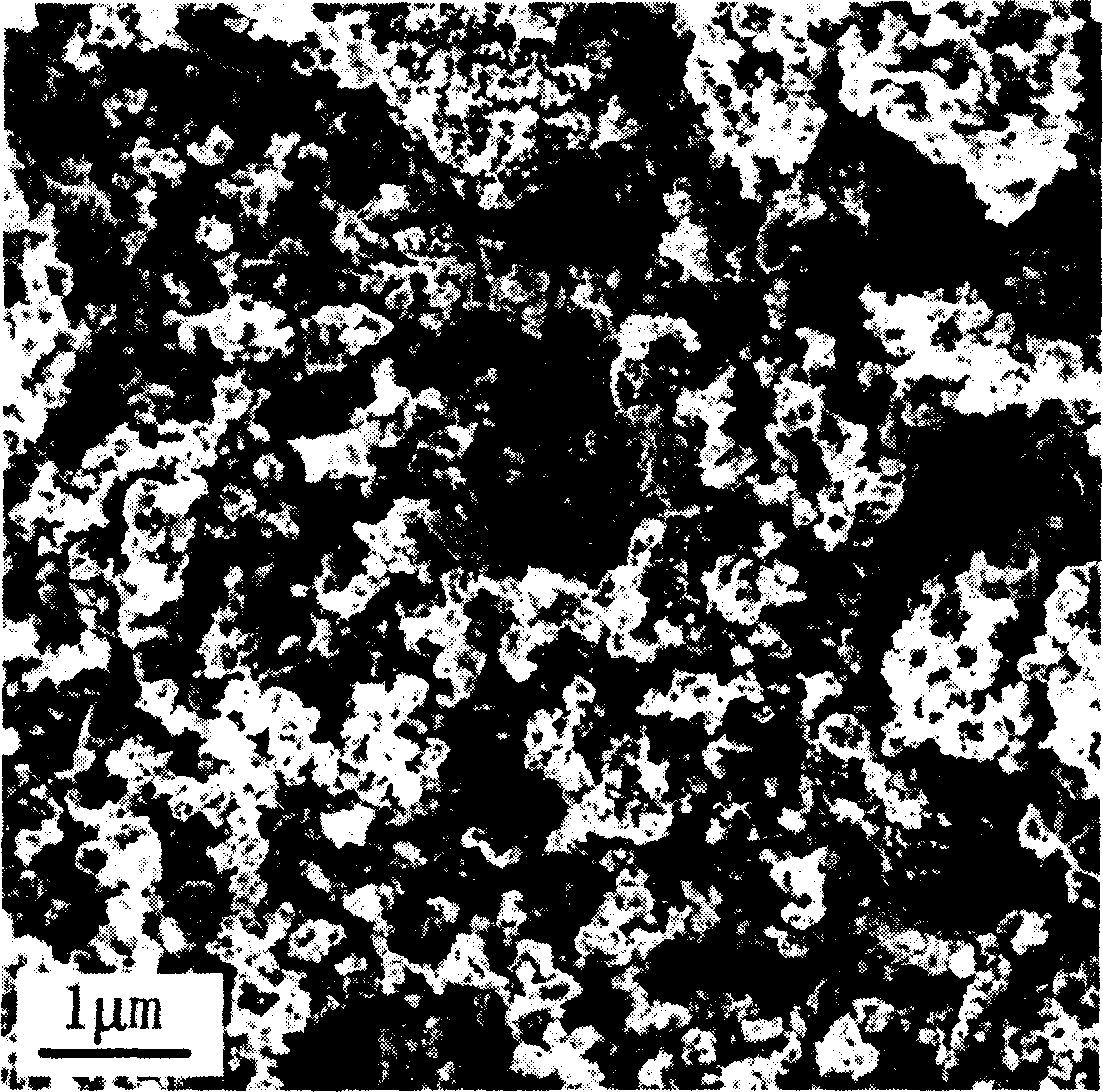
[0030] The XRD pattern of the product is shown in figure 1 In the curve a, it can be seen from the figure that the pure-phase monoclinic lithium vanadium phosphate positive electrode material is synthesized by using the solid-state sintering method. There is no impurity peak in the spectrum, and the product has high purity. The scanning electron micrograph of the product is...
Embodiment 2
[0032] LiNO 3 、VO 2 and (NH 4 ) 2 HPO 4 Weigh 50 g according to the stoichiometric ratio of Li: V: P = 3.06: 2: 3, mix them uniformly, and ball mill them on a planetary ball mill for 12 hours; then carry out pretreatment at 200° C. for 8 hours in an air atmosphere, and after natural cooling, obtain Powdered product; then add 10 g of acetylene black to the above powdered product as a carbon source, ball mill again in a planetary ball mill for 24 hours, and sinter at 500 °C for 8 hours in an Ar gas atmosphere under a pressure of 10 MPa to obtain a carbon-coated Lithium vanadium phosphate cathode material.
[0033] The XRD pattern of the product is shown in figure 1 In the middle b curve, it can be seen from the figure that there is no impurity in the product, and it is a pure-phase monoclinic lithium vanadium phosphate positive electrode material. The first charge and discharge curve of the lithium vanadium phosphate positive electrode material is shown in Figure 5 , the...
Embodiment 3
[0035] LiCH 3 COO, V 2 o 3 and (NH 4 ) 3 PO 4 Weigh 100g according to the stoichiometric ratio of Li:V:P=3.1:2:3, mix it evenly, and ball mill it on a planetary ball mill for 20h; then under air atmosphere, carry out pretreatment at 400°C for 12h, after natural cooling, to obtain Powdered product; then add 1 g of graphite to the above powdered product as a carbon source, ball mill again in a planetary ball mill for 20 hours, and sinter at 600°C for 2 hours in a He gas atmosphere to obtain carbon-coated phosphoric acid Lithium vanadium cathode material.
[0036] The XRD pattern of the product is shown in figure 1 In the curve c, it can be seen from the figure that there is no impurity in the product, and it is a pure-phase monoclinic lithium vanadium phosphate positive electrode material. The first charge and discharge curve of the lithium vanadium phosphate positive electrode material is shown in Figure 6 , when the charging and discharging voltages are 3-4.3V, 3-4.5V...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com