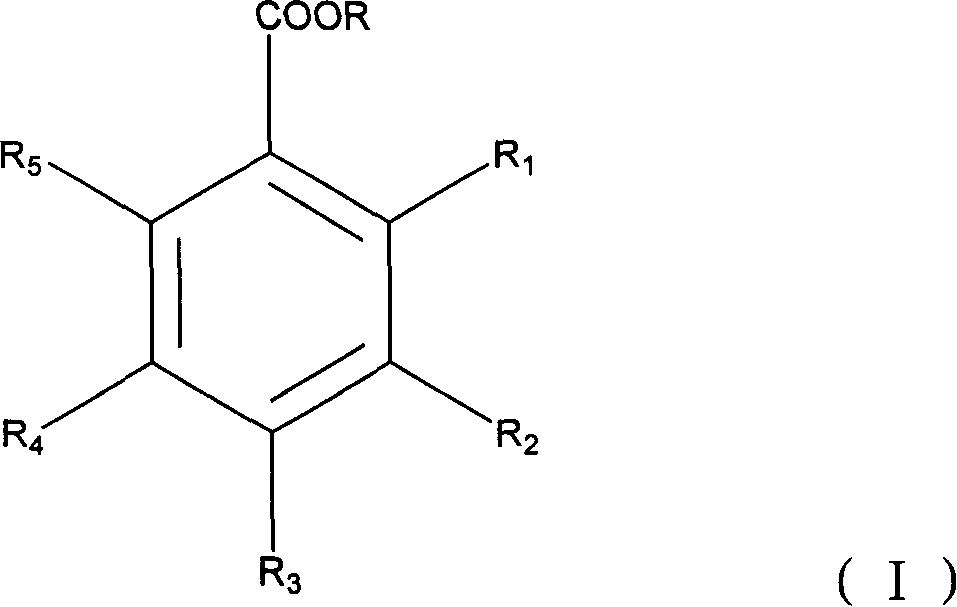
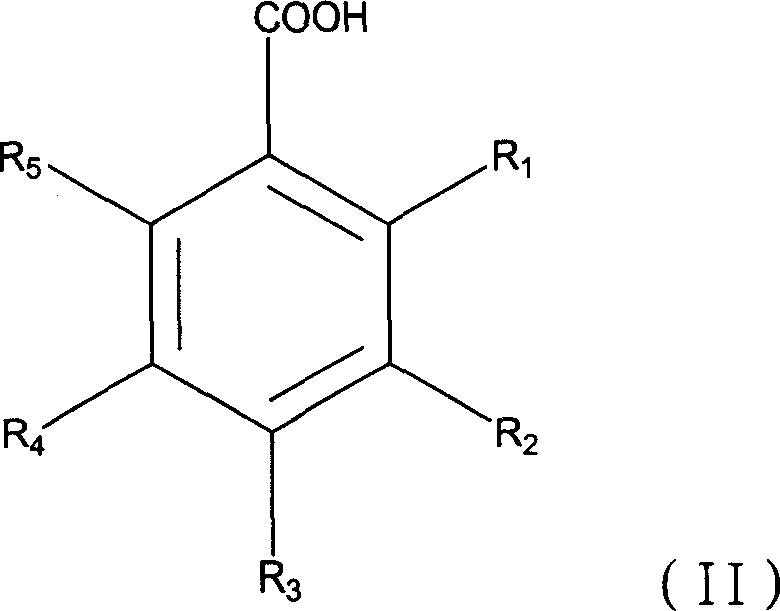
Application of hydroxybenzoate acid and its analogue in the preparing process of medicine for preventing and treating virus infection
A technology of hydroxybenzoate and trihydroxybenzoate, which is applied in the field of application of hydroxybenzoate and its analogs in the preparation of drugs for the prevention and treatment of viral infections, and can solve the problem of high toxicity and long-term Medication, short-term application only has temporary effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 anti-HPV virus microemulsion
[0048] Take 20ml of 1,2-propanediol, 15ml of Tween 80, and 5ml of azone, mix them, add sterilized distilled water until the total volume is 100ml, and obtain an external preparation solution. Take benzoic acid, salicylic acid, benzoic acid + salicylic acid, p-hydroxybenzoic acid, 2,3-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, p-hydroxybenzoic acid Ethyl formate, methyl 3,4-dihydroxybenzoate, gallic acid, lauryl gallate, octyl gallate, propyl gallate, ethyl gallate, methyl gallate, 2,4-bis Each 10g of methyl hydroxybenzoate, respectively add external preparation solution 50ml, adjust pH to 5.5, then add external preparation solution to 100ml, get 10% anti-HPV virus microemulsion of the present invention and control group microemulsion.
Embodiment 2
[0049] Example 2 Preparation of anti-HBV virus propyl gallate injection
[0050] Take 10g of propyl gallate, 8.5g of sodium chloride, 10ml of 1,2-propanediol, and 10ml of Tween 80, mix them, add sterilized distilled water to dissolve, and add sterilized distilled water to 100ml, adjust the pH to 7.4, filter , potted, and sterilized at 100°C for 30 minutes. Propyl gallate injection was obtained.
Embodiment 3
[0051] Example 3 Preparation of Compound Ethyl Gallate Effervescent Tablets
[0052] Take 0.25g of ethyl gallate, 0.01g of polymyocytes, and 0.45g of tartaric acid to pass through a 80-mesh sieve respectively, make a soft material with 95% ethanol, pass through a 12-mesh sieve to make wet granules, dry at 50°C, and set aside. Separately take 0.65g of sodium bicarbonate, 0.02g of dextrin and add sterile distilled water to make soft materials, pass through a 12-mesh sieve to make wet granules, dry at 50°C, then mix with the above-mentioned dry granules, granulate, add appropriate amount of sterilized distilled water, and dry After a while, add 0.01g PEG6000. Mix well, press into tablets, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com