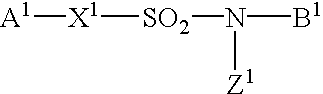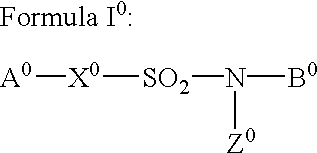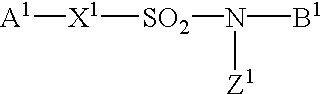Sulfonamide derivatives
a technology of sulfonamide and derivatives, which is applied in the direction of amide active ingredients, heterocyclic compound active ingredients, biocide, etc., can solve the problems of resistance to microorganisms and no sulfonamide derivatives have been developed, and achieve excellent microorganismicidal effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0576] Synthesis of 2',4'-Dichloro-N-methyl-p-toluenesulfonanilide
[0577] (Compound No. 51)
[0578] To a suspension of sodium hydride(60%, 0.07g (1.75 mmol)) in DMF (2.0 ml), 2',4'-dichloro-p-toluenesulfonanilide(0.50 g (1.58 mmol)) was added with stirring at room temperature. To the resulting mixture, after 15 minutes' stirring at room temperature, dimethyl sulfate(0.17 ml (1.80 mmol)) was added dropwise. After 15 hours' stirring at room temperature, the reaction mixture was poured into water and extracted with ethyl acetate. The extract was washed with water and saturated sodium chloride solution, successively, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. The crystals separated were filtered and washed with diisopropyl ether to give 0.38 g (73%) of the title compound.
[0579] mp: 86.0-87.0.degree. C.
[0580] NMR(CDl.sub.3) .delta.: 2.44(3H,s), 3.17(3H,s), 7.13(1H,d,J=8.6 Hz), 7.22(1H,dd,J=8.6& 2.3 Hz), 7.30(2H,d,J=8.4 Hz), 7.42(1H,d,J=2.3 Hz), 7.6...
example 2
[0581] Synthesis of N,4'-Dimethyl-3'-nitro-p-toluenesulfonanilide
[0582] (Compound No. 126)
[0583] To a suspension of sodium hydride (60%, 0.14g (3.50 mmol)) in DMF (3.0 ml), 4'-methyl-3'-nitro-p-toluenesulfonanilide (1.00 g (3.26 mmol)) was added with stirring at room temperature. To the resulting mixture, after 15 minutes' stirring at room temperature, dimethyl sulfate (0.34 ml (3.56 mmol)) was added dropwise. After one hours' stirring at room temperature, the reaction mixture was poured into water and extracted with ethyl acetate. The extract was washed with water and saturated sodium chloride solution, successively, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. Diethyl ether was added to the residue, and the solid separated was filtered and washed to give 0.79 g (76%) of the title compound as pale yellow crystals.
[0584] mp: 103.5-106.0.degree. C.
[0585] NMR(CDCl.sub.3) .delta.: 2.43(3H,s), 2.59(3H,s), 3.17(3H,s), 7.27(2H,d,J=8.3 Hz), 7.31(1H,...
example 3
[0586] Synthesis of N-Ethyl-4'-fluoro-2'-nitro-p-toluenesulfonanilide
[0587] (Compound No. 263)
[0588] To a suspension of sodium hydride (60%, 0.05 g (1.25 mmol)) in DMF (2.0 ml), 4'-fluoro-2'-nitro-p-toluenesulfonanilide (0.31 g (1.00 mmol)) was added with stirring at room temperature. To the resulting mixture, after 15 minutes' stirring at room temperature, ethyl iodide (0.30 ml (3.75 mmol)) was added dropwise. After 30 minutes' heating at 100.degree. C. with stirring, the reaction mixture was poured into water and extracted with ethyl acetate. The extract was washed with water and saturated sodium chloride solution, successively, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. Diisopropyl ether was added to the residue and the solid separated was filtered and washed to give 0.25 g (74%) of the title compound as pale yellow crystals.
[0589] mp: 93.0-94.5.degree. C.
[0590] NMR(CDCl.sub.3) .delta.: 1.18(3H,s), 2.44(3H,s), 3.67(2H,brq,J=7.2 Hz), 7.10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific gravity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com