Methods for surface modification
a surface modification and surface technology, applied in the field of surface modification, can solve the problems of poor adhesion, printability, adaptability of the surface for coating, and limited success of methods, and achieve the effects of preventing uniform property characteristics, affecting the function of materials, and high control and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
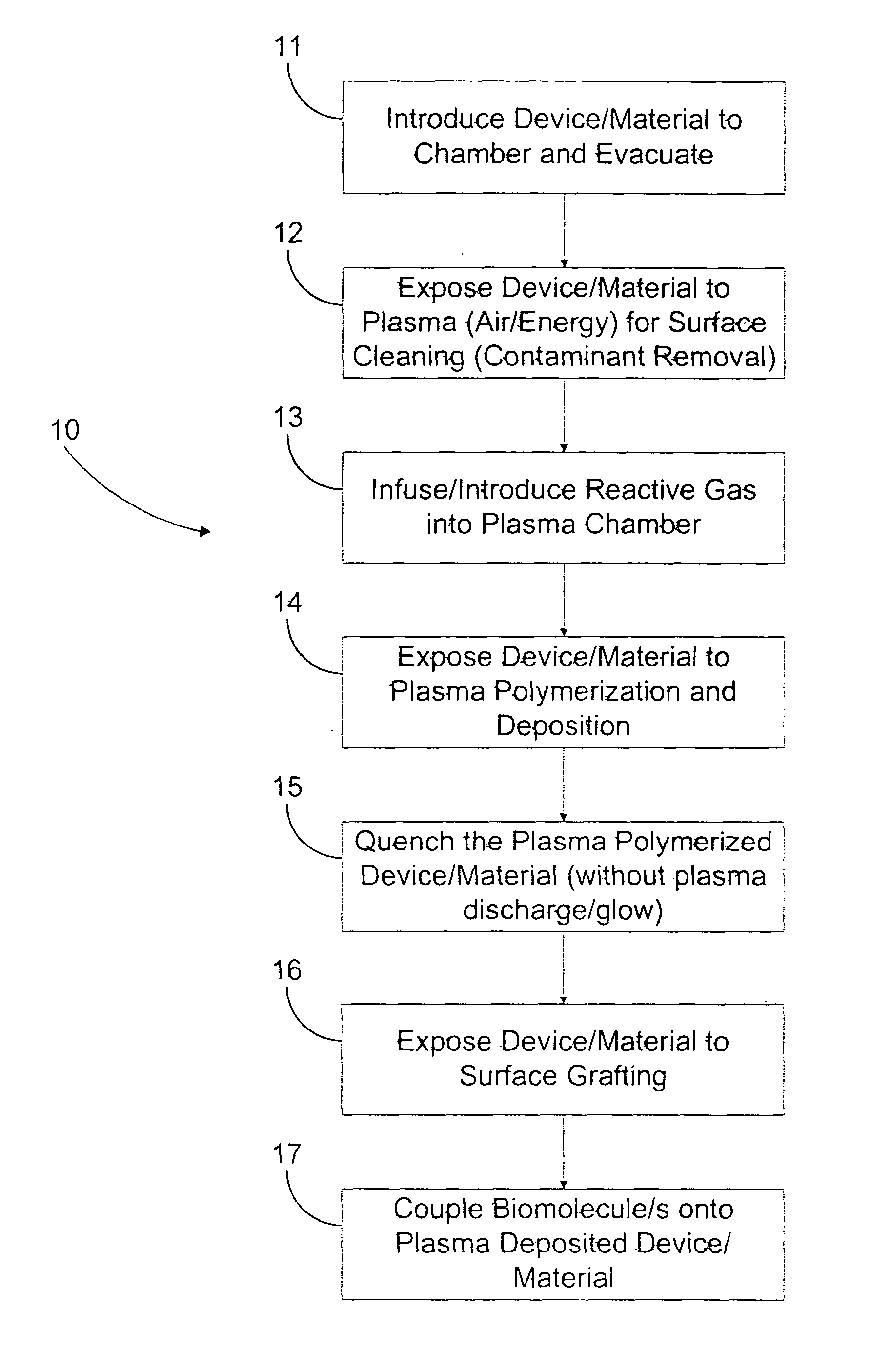
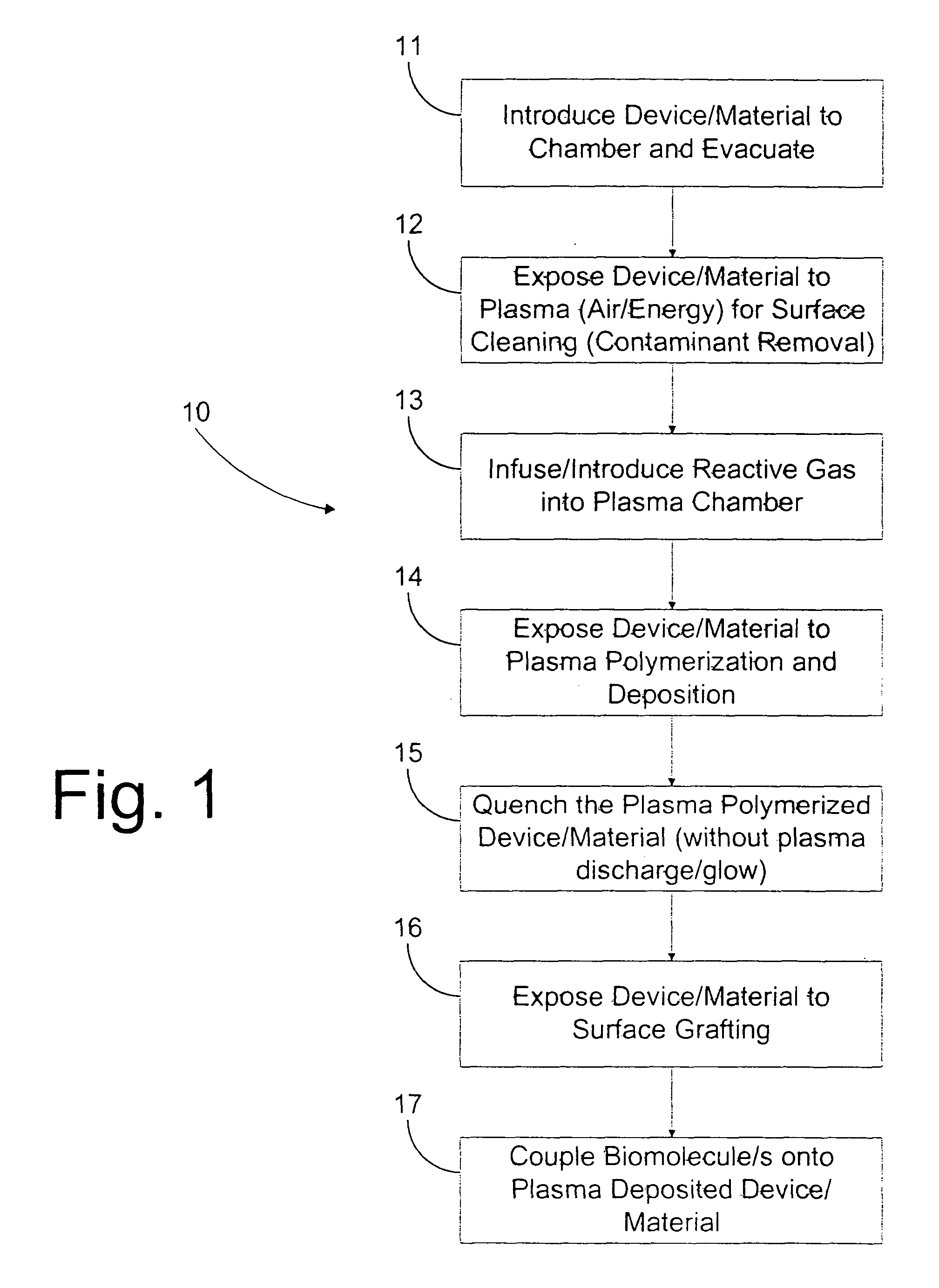
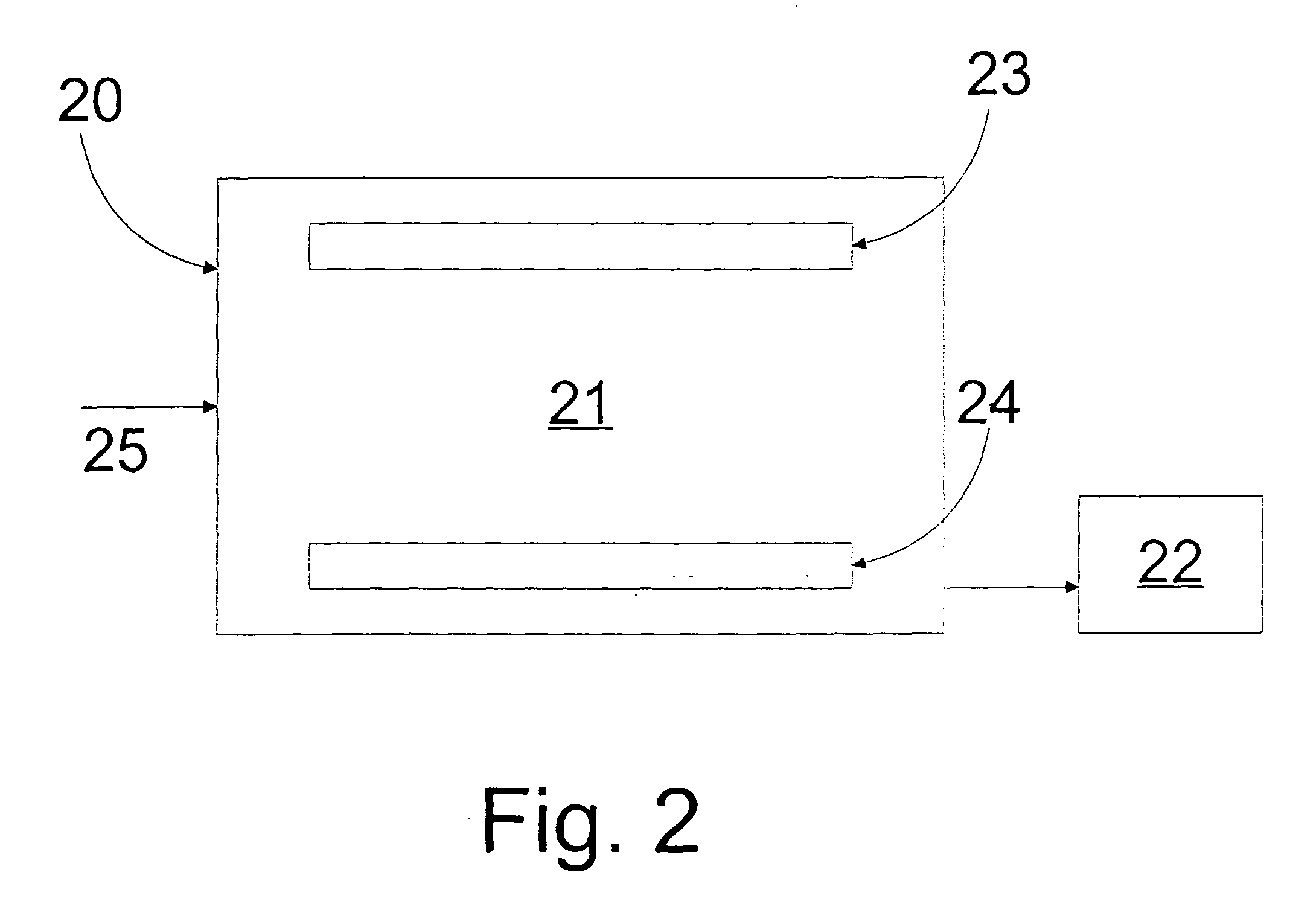
Image
Examples
example 2
[0059] Copolymer Grafting of Stents
[0060] In another embodiment of this invention, copolymerization grafting was performed on stents. The stents were initially pre-treated with plasma as generally described above in Steps 12-15. Then, to prepare a grafting solution, 70 g of a solution containing 35% distilled acrylic acid added to 120 g of deionized water to which 10 g of acrylamide had been dissolved. The resultant solution was then placed in a 300 mL glass vessel. After 2 minutes of stirring, argon gas was introduced with a slight bubbling into the solution. After 10 minutes, 6 ml of CAN (cerric ammonium nitrate) catalyst / initiator was added and allowed to stir with bubbling Argon for another 2 minutes after which the argon was discontinued. The premixed grafting solution was slowly dispensed into 10 ml glass tubes. The plasma-treated and plasma deposited stents were immersed into the solution and placed in an ultrasonic water bath (temp. about 18-25 degrees C.). The total graftin...
example 3
[0064] Copolymer Grafting of the Present Method v. Other Methods
[0065] A study was performed to compare three sets of e-PTFE covered stents: the first group was subject to a preferred embodiment of the method of the present invention; the second group was subject to another known bioactive surface treatment method; and the third group (control) was not subject to any surface treatment.
[0066] Embodiment of Method of the Present Invention
[0067] The first group was subject to an embodiment of the method of the present invention substantially described in Example 2 above with some modification. The stents were initially cleaned by being subject to 1 minute of air plasma at 50 W and 20 sccm air flow rate into the plasma chamber. Next, the stents were subject to plasma deposition for 5 minutes under propylene plasma, at 50 W and 110 sccm propylene flow rate into the plasma chamber. A quenching period of 30 seconds followed the plasma deposition, wherein the electrodes were not activated, ...
example 4
[0078] Surface Deposition of Adhesion Molecules
[0079] Collagen exhibits excellent cell adhesion properties, promotes natural wound healing, and stimulates fibroblast adhesion and growth. Thus, it would be beneficial to deposit collagen upon surfaces of certain medical devices to promote incorporation of the device into the body tissues. The present inventors have discovered that collagen may be covalently bonded to an acrylic acid (AA) substrate surface. Devices that have collagen grafts exhibit excellent cell adhesion properties.
[0080] As an example of collagen grafting, the present inventors used glass slides to provide a method for grafting collagen onto a material. First, acrylic acid (AA) grafted slides were prepared as generally described above, and further subjected to collagen coupling. Collagen was supplied (by Biophil Chimica Fine srl, Vimodrone (MI), Italy) as a 1% collagen native solution. This is a soluble collagen obtained from fresh calf skin. The extraction is done v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com