Preparation and use of biofilm-degrading, multiple-specificity, hydrolytic enzyme mixtures
a technology of hydrolytic enzyme and biofilm, which is applied in the direction of detergent compounding agents, peptide/protein ingredients, and cleaning compositions, etc. it can solve the problems of increased maintenance, increased efficiency of industrial machinery, and potential health hazards, so as to improve hygiene, reduce biofilm, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
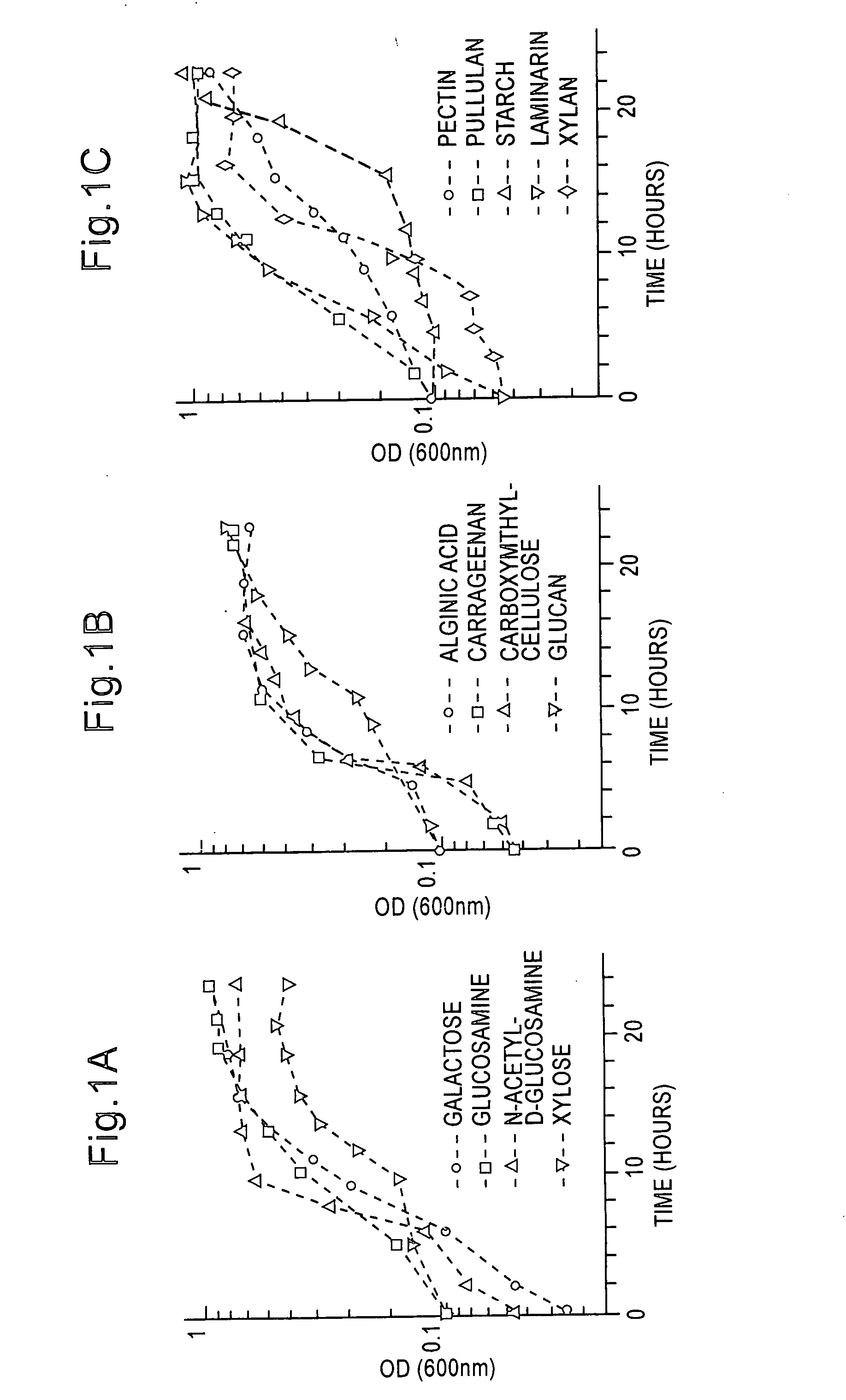
Effect of Sole Carbon Sources on the Production of Carbohydrases
[0045] Media, chemicals and growth parameters. To assess the production of carbohydrases when using various carbon sources, 2-40 was grown in minimal media (Table 1) containing a final concentration of 0.2% of one of the following carbon sources: agar or its degradation products (neoagarotetraose, neoagarobiose, D-galactose), alginic acid, carrageenan, carboxymethyl cellulose, colloidal chitin or its degradation products (chitobiose, chitotriose, N-acetyl-D-glucosamine), D-glucose (previously reported to repress the Microbulbifer 2-40 agarase system and other bacterial chitinase systems) (Stosz, 1994; Frändberg & Schnürer, 1994), laminarin (determined to repress chitinase systems in other bacteria) (Frändberg & Schnürer, 1994), -glucan (determined to repress other bacterial chitinase systems) (Frändberg & Schnürer, 1994), pectin (determined to induce other bacterial chitinase systems) (Frändberg & Schnürer, 1994), pull...
example 2
Production and Purification of Enzyme Systems
[0055] Chemicals, media and bacterial growth conditions. Pseudomonas atlantica agarase (Sigma Chemical Co., St. Louis, Mo.) and chitinase, harvested from Vibrio harveyi, served as positive controls in zymograms. Broth media was prepared as described in Example 1 (Table 1). Cultures were also grown on solid media. Solid agar plates were made by adding 1% agar to the MM broth recipe (Table 1).
[0056] To induce chitinase production without agarase production, 2-40 was cultured on MM plates containing a purified chitin paste and were hardened with phytagel (Table 2.1). Chitin paste was purified from commercial chitin as outlined by Liggappa and Lockwood (1962). Practical grade chitin was soaked in 1 M NaOH for 24 hours. After the chitin was washed with dH2O, it was soaked in 1 M HCl for 24 hours, washed again with dH2O, and transferred to 1 M NaOH. The alternate base / acid soaking step was repeated four times as described. Following the final...
example 3
Production of Filamentous Tubules
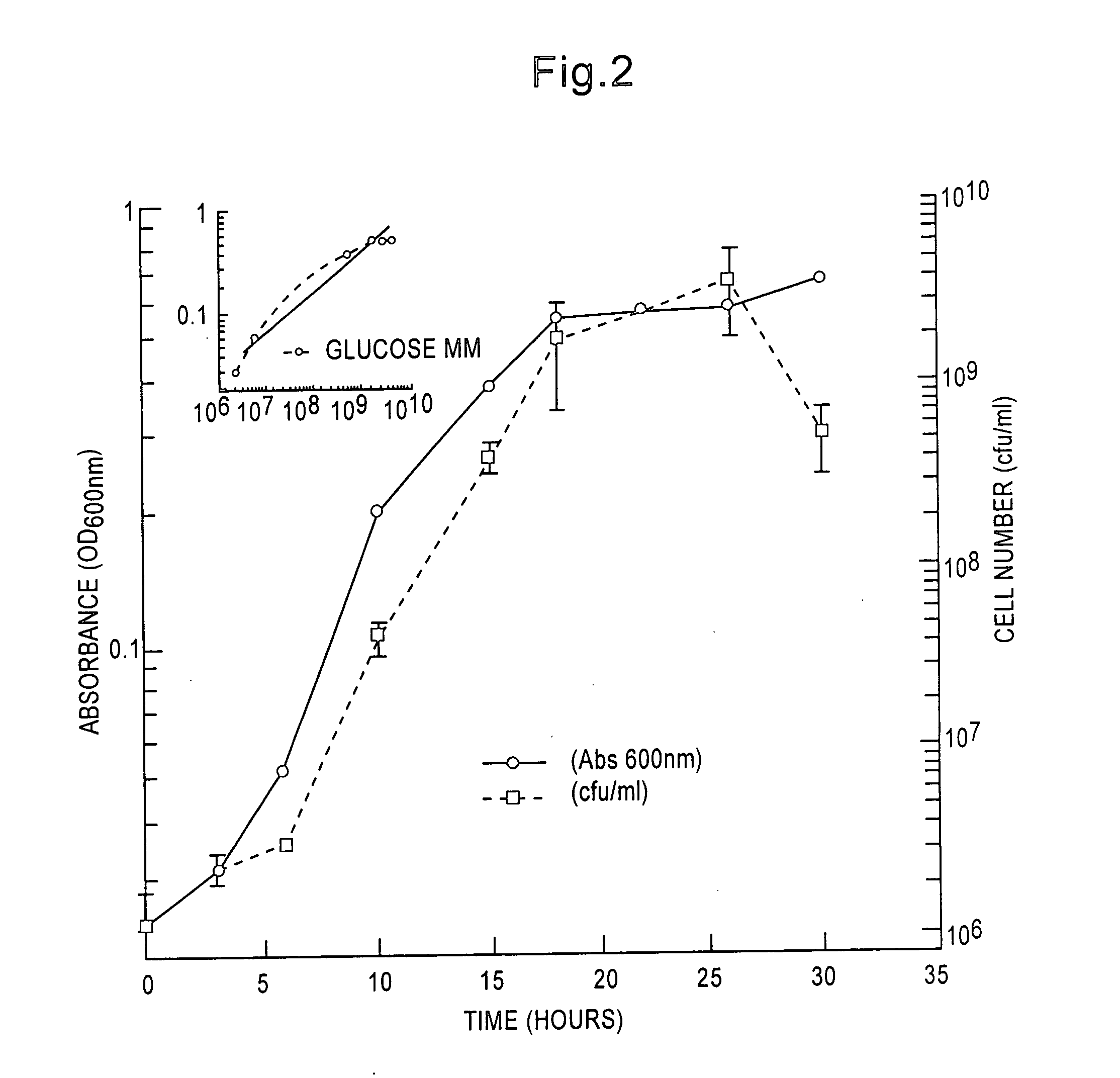
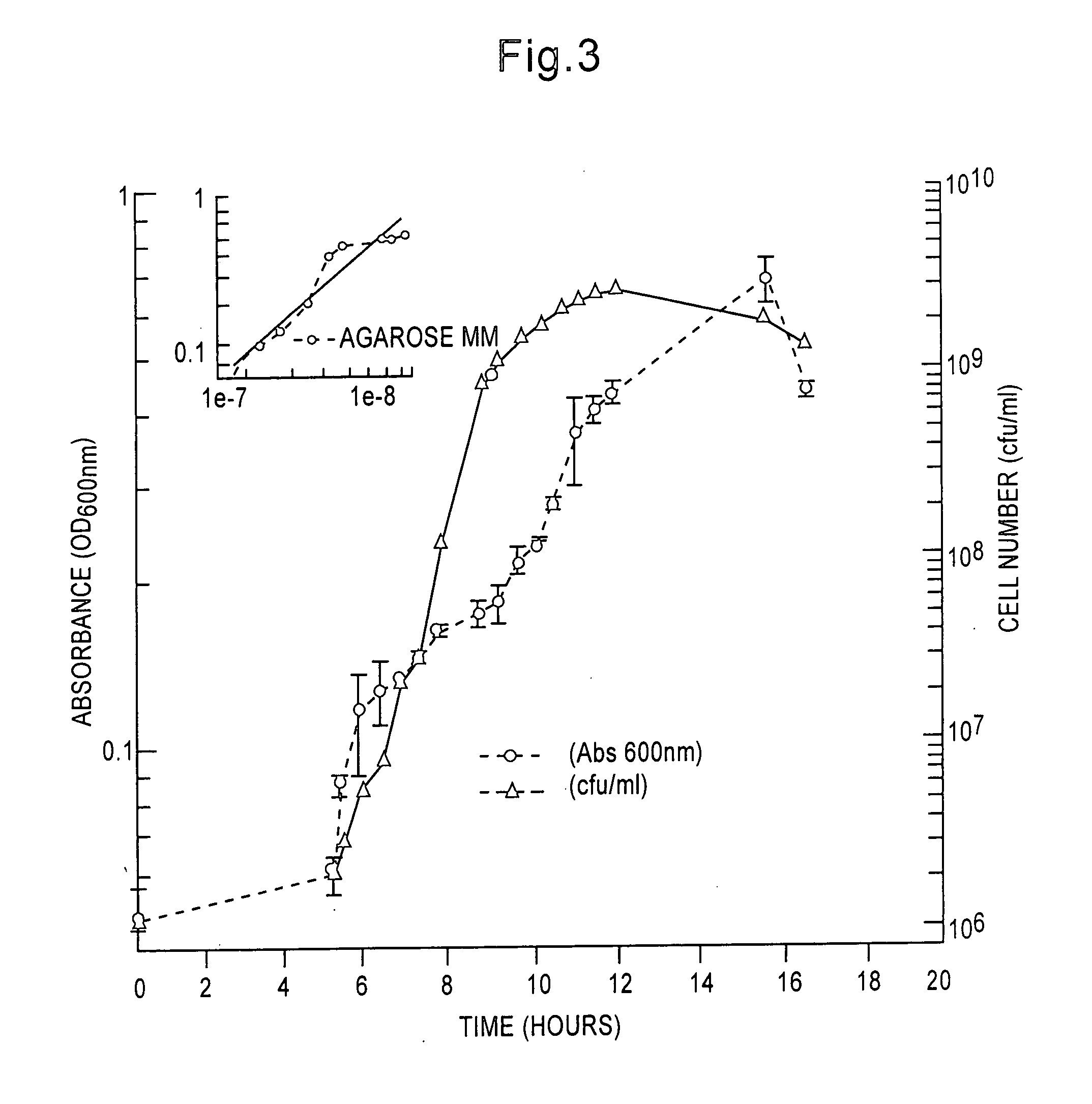
[0077] Morphogenesis in sole and multiple carbon source MM. Microbulbifer 2-40 cells grown in glucose MM have smooth surfaces during early logarithmic phase growth. Bleb-like vesicles were formed during mid-log through stationary culture phases. Vesicles were formed due to separation of the inner and outer membrane of the cell. (These vesicles eventually partition from the cell body being released in late culture stages). During late culture phases in glucose MM, late stationary to death phase, an abundance of long, filamentous tubules, coated with small nodules, were synthesized. The tubules were −20-50 nm in diameter and their length extended up to several micrometers. The nodules have an approximate diameter of 20-40 nm.
[0078] In addition to degradosomes, filamentous tubules and bleb-like vesicles were produced during logarithmic phase growth in MM containing agar or chitin. The appearance and abundance of tubules and blebs during early growth s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com