Methods of preforming homologous recombination based modification of nucleic acids in recombination deficient cells and use of the modified nucleic acid products thereof
a technology of recombination deficiency cells and homologous recombination, which is applied in the direction of peptides, applications, peptide sources, etc., can solve the problems of critical limitations in the yac system, the size of the transgene is presently limited, and the use of such large genomic transgenes has several practical problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Homologous Recombination Based Modification in E. coli and Germline Transmission in Transgenic Mice of an 131 Kilobase Bacterial Artificial Chromosome
Introduction
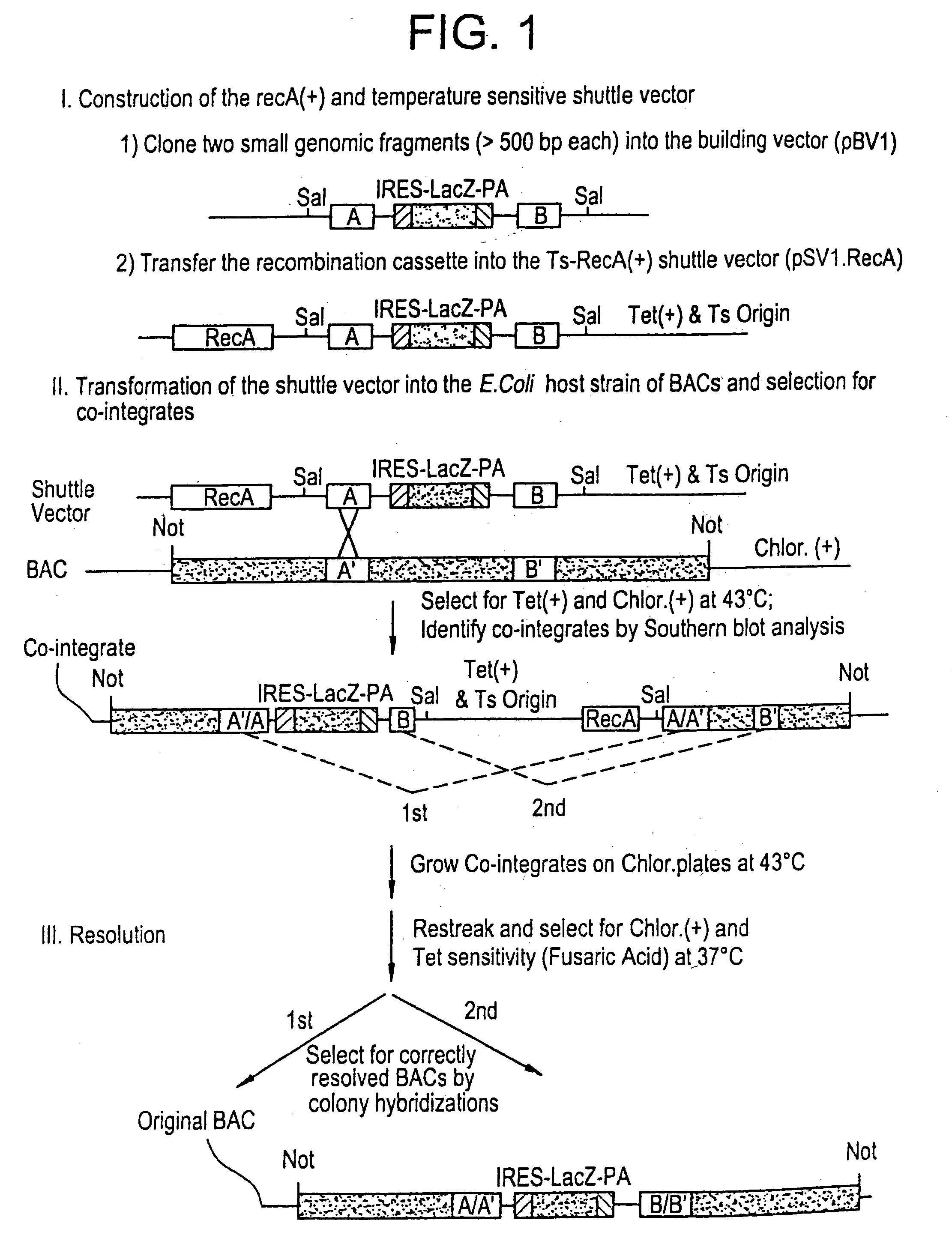
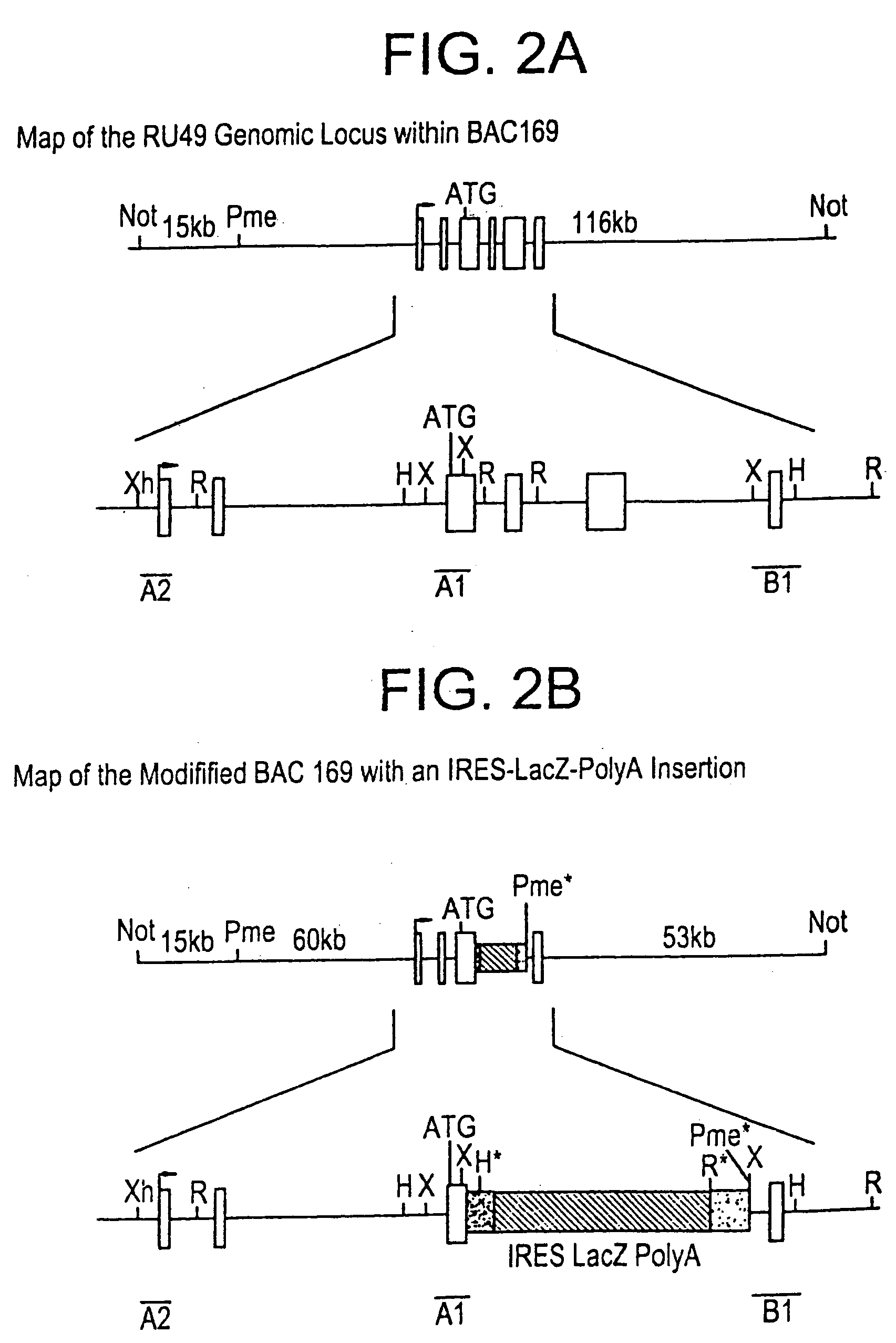
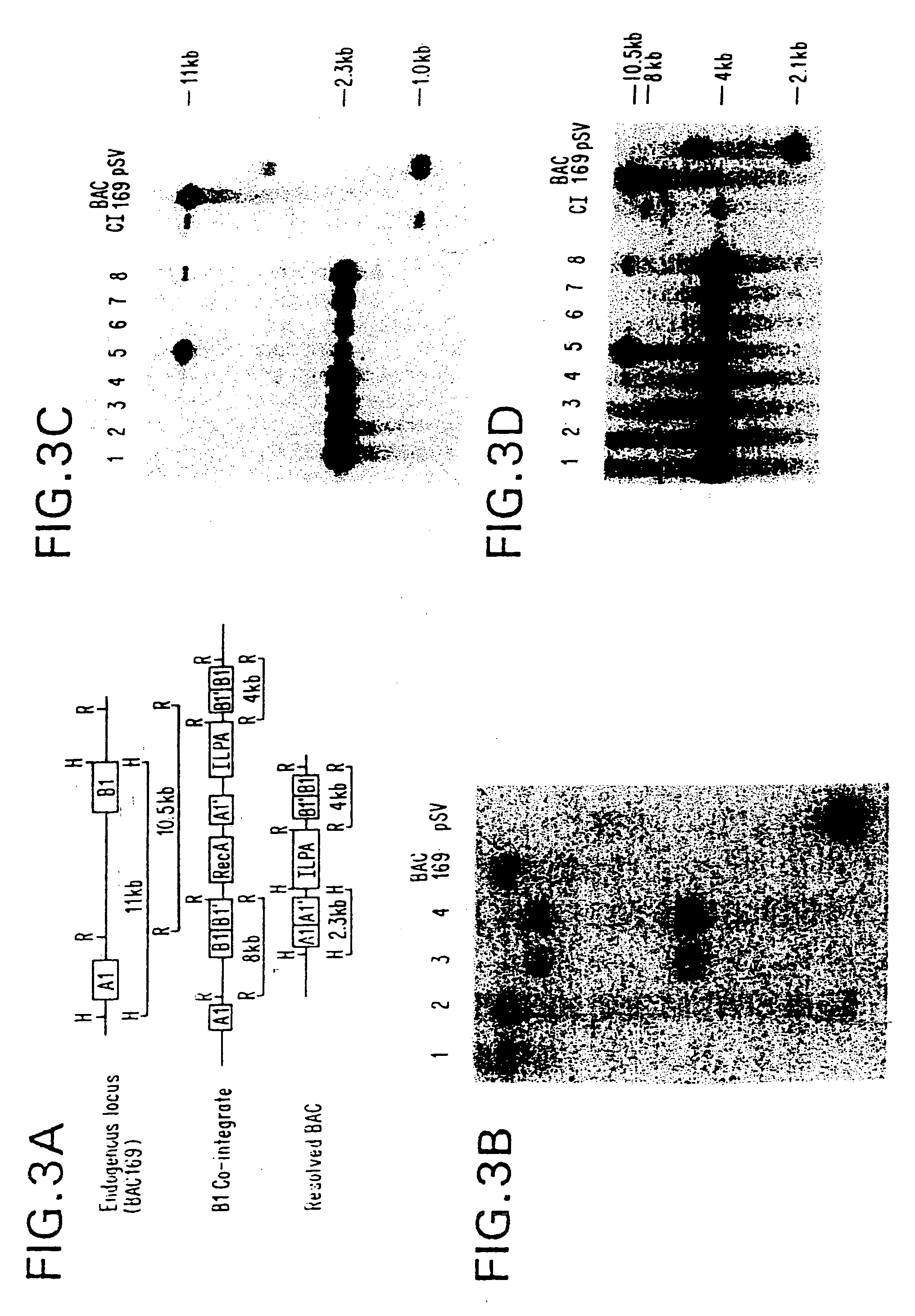
[0209] Bacterial based artificial chromosomes, such as Bacterial artificial chromosomes (BACs) and P-1 derived artificial chromosomes (PACs), are circular bacterial plasmids that may propogate as large as 300 kb of exogenous genomic DNA [Shizuya et al., PNAS, 89:8794-8797, (1992); Ioannou et al., Nature Genet., 6:84-90 (1994)]. For the majority of BAC and PAC libraries, the average size of the insert is 130-150 kb. There are several advantages of using bacterial based artificial chromosomes for genomic and functional studies, compared to the yeast based system (i.e. YACs): First, BAC and PAC libraries are much easier to construct due to higher cloning efficiency. Second, BACs and PACs ate propagated in recombination deficient E. coli host cells, so they have high stability and minimal chimerism. No rearrangements have bee...
example 2
BAC Mediated Gene Dosage Analysis: A Role for Zipro1 (RU49 / Zfp38) in Progenitor Cell Proliferation in Cerebellum and Skin
Introduction
[0331] Analysis of loss-of-function phenotypes has played a central role in the discovery of complex morphogenetic pathways in a variety of organisms. For example, the seminal loss-of-function screens for genes affecting cell cycle traverse in yeast [Hartwell et al, Science 183(120):46-51 (1974)] and for mutations affecting early D. melanogaster development [Nusslein-Volhard and Wieschaus, Nature 287:795-801 (1980)] have provided the basis for our current understanding of cell division and of embryonic patterning. However, in both cases, it was readily appreciated that loss-of-function genetics would not yield all the genes in the pathway under study, and alternative strategies for genetic analysis were therefore devised. Thus, high copy number suppression screens have been highly successful in identifying additional genes important for cell division...
example 3
Rapid Modification and High Throughput Resolution of Bacterial Artificial Chromosomes in Liquid
Introduction
[0364] Traditionally, overexpression of cDNA in eukaryotic cells and transgenic mice has been widely used for the study of gene function and regulation. However, the cDNA itself is often missing important elements for regulation of gene activity, such as high-level, tissue-specific, and integration site-independent expression of the transgene. Those elements such as enhancers, locus control regions (LCR), and insulators, may reside at a large distance (>50 kb) from the gene itself. A intact genomic loci as a transgene will be essential for this expression. Bacterial artificial chromosome (BACs) and P-1 derived artificial chromosomes (PACs) have become a widespread and powerful resource in manipulating the large genomic DNA in E. coli. However, although BAC transgenic technology has been used for studying gene function and regulation, the efficiency for modifying and resolving...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
| Recombination enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com