Elastin prevents occlusion of body vessels by vascular smooth muscle cells
a technology of vascular smooth muscle cells and elastin, which is applied in the direction of biological material analysis, dna/rna fragmentation, enzymes, etc., can solve the problems of ineffective incite inflammation in the vessel wall, and inability to achieve the effect of animal models or clinical settings, and achieves the effect of increasing the f:g actin ratio, and increasing the gtp-bound (
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Elastin Regulates the Contractile Phenotype of Vascular Smooth Muscle Cells
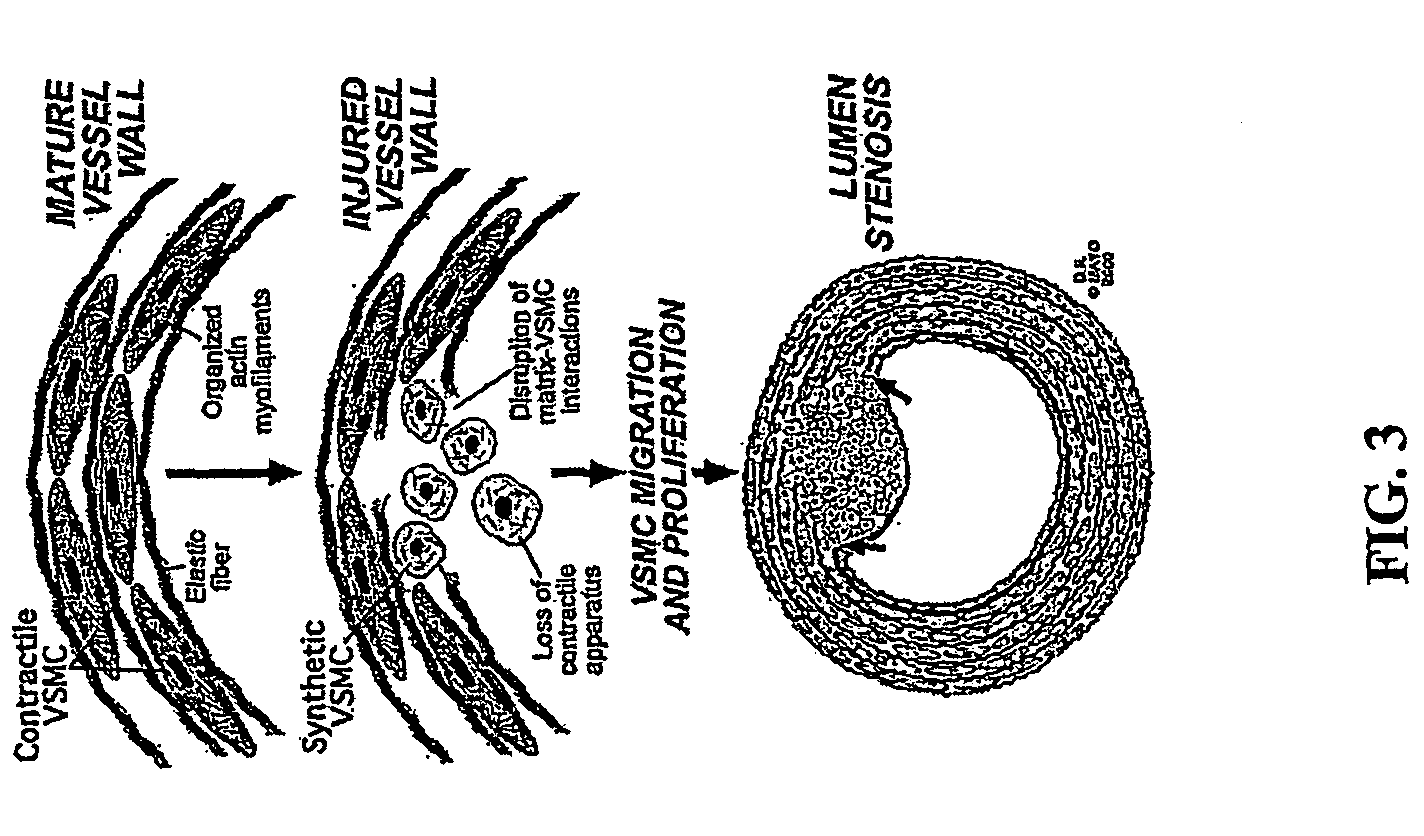
[0317] Tropoelastin, the gene product of the elastin gene, is the major extracellular matrix protein in an artery and is synthesized and deposited by vascular smooth muscle cells (vsmcs). Mature arterial smooth muscle cells exist in a quiescent state specialized for contraction, with highly organized actin stress fibers. In response to vascular insults, these cells change their phenotype, lose their actin stress fibers, proliferate and migrate into the arterial lumen. Elastin signaling regulates the phenotypic modulation of vascular smooth muscle cells from a quiescent contractile state to a proliferative non-contractile state. As demonstrated herein, the elastin receptor is not a member of the integrin family of matrix receptors (divalent cation chelator insensitive) and is a G protein coupled receptor (pertussis toxin sensitive) that signals through both Gi and Gs families of heterotrimeric receptors.
[031...
example 2
Tropoelastin Modulates Migration of Vascular Smooth Muscle Cells
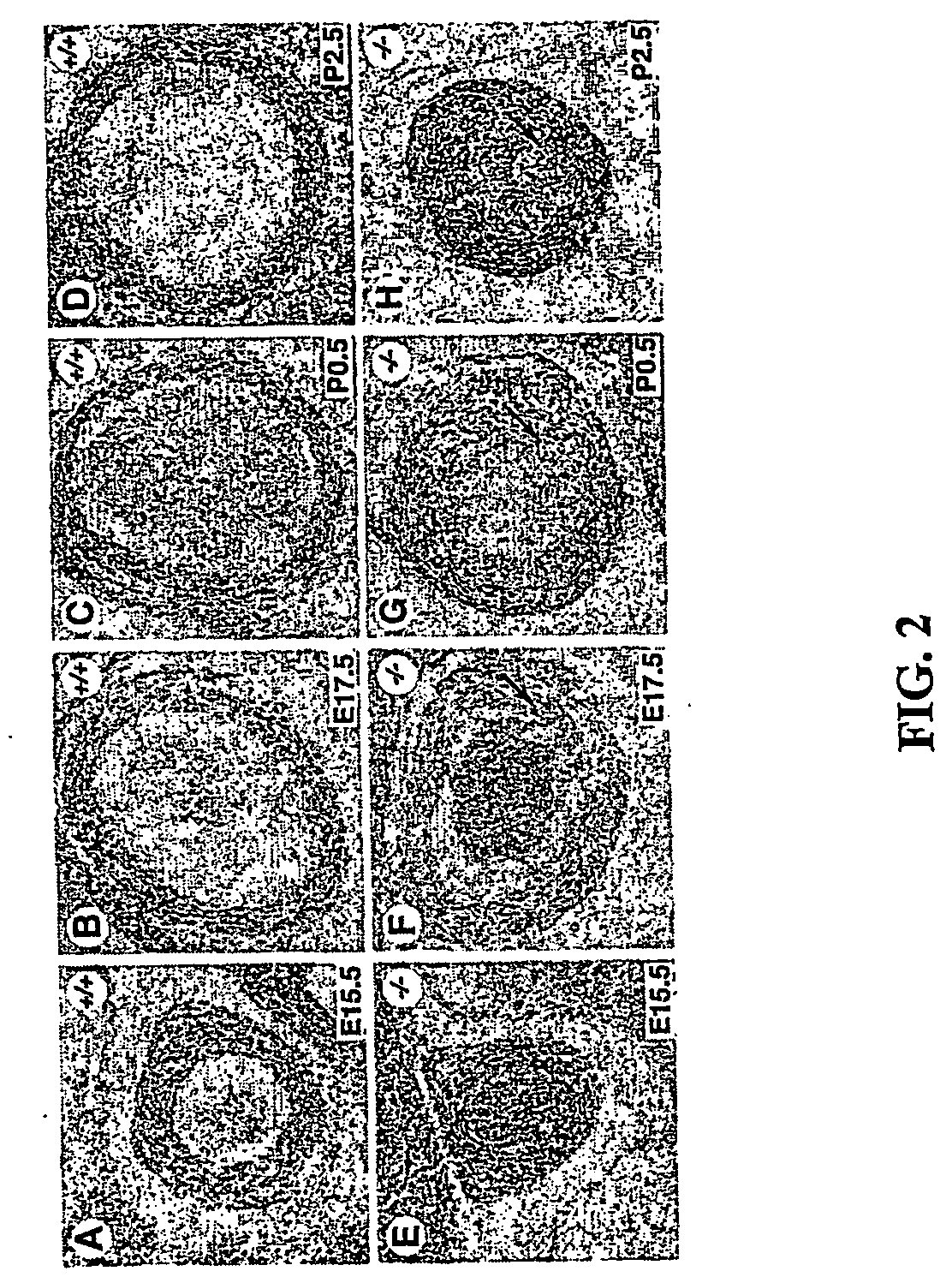
[0321] In Eln − / − arteries, vascular smooth muscle cells accumulated in the subendothelial space and occluded the artery. These data suggested that the elastic lamellae localize vsmcs and regulate their proliferation. Because the elastic lamellae are highly fenestrated allowing cells to migrate through, it is unlikely that elastin simply forms a physical cage that contains vsmcs. Instead, we postulated that elastin signals vsmcs to migrate and adhere to the elastic lamellae. To test this hypothesis we used a modified Boyden chamber assay (Li et al., 1998; Raines et al., 1993). This assay system consists of two adjacent chambers separated by a semipermeable membrane. The amount of cells placed in one chamber (upper) that migrate to the other chamber (lower) is measured by counting the number of cells that have passed through to the undersurface of the filter.
[0322] Our migration data showed three important results. Fir...
example 3
Elastin Signals Through a G-Protein Coupled Receptor
[0324] G-protein coupled receptors (GPCRs) are the largest family of transmembrane receptors that are activated by ligands including amino acids, peptides, nucleotides, lipids, retinal and phermones. Examples of such receptors include the adrenergic receptors. Ligands binding to GPCRs cause a conformational change and activate intracellular heterotrimeric G proteins (α, β and γ subunits). The effector G proteins are classified into four protein families based on composition of the α-subunit: Gs, Gi, Gq / 11, and Golf. Each class of effector G-proteins activates distinct downstream biochemical pathways. The availability of unique inhibitors and biochemical markers specific for each class of G-proteins allows the dissection of the molecular nature of signaling in response to a particular ligand. All GPCRs regardless of which G protein they activate share a common structure of seven transmembrane domains.
[0325] In order to dissect the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com