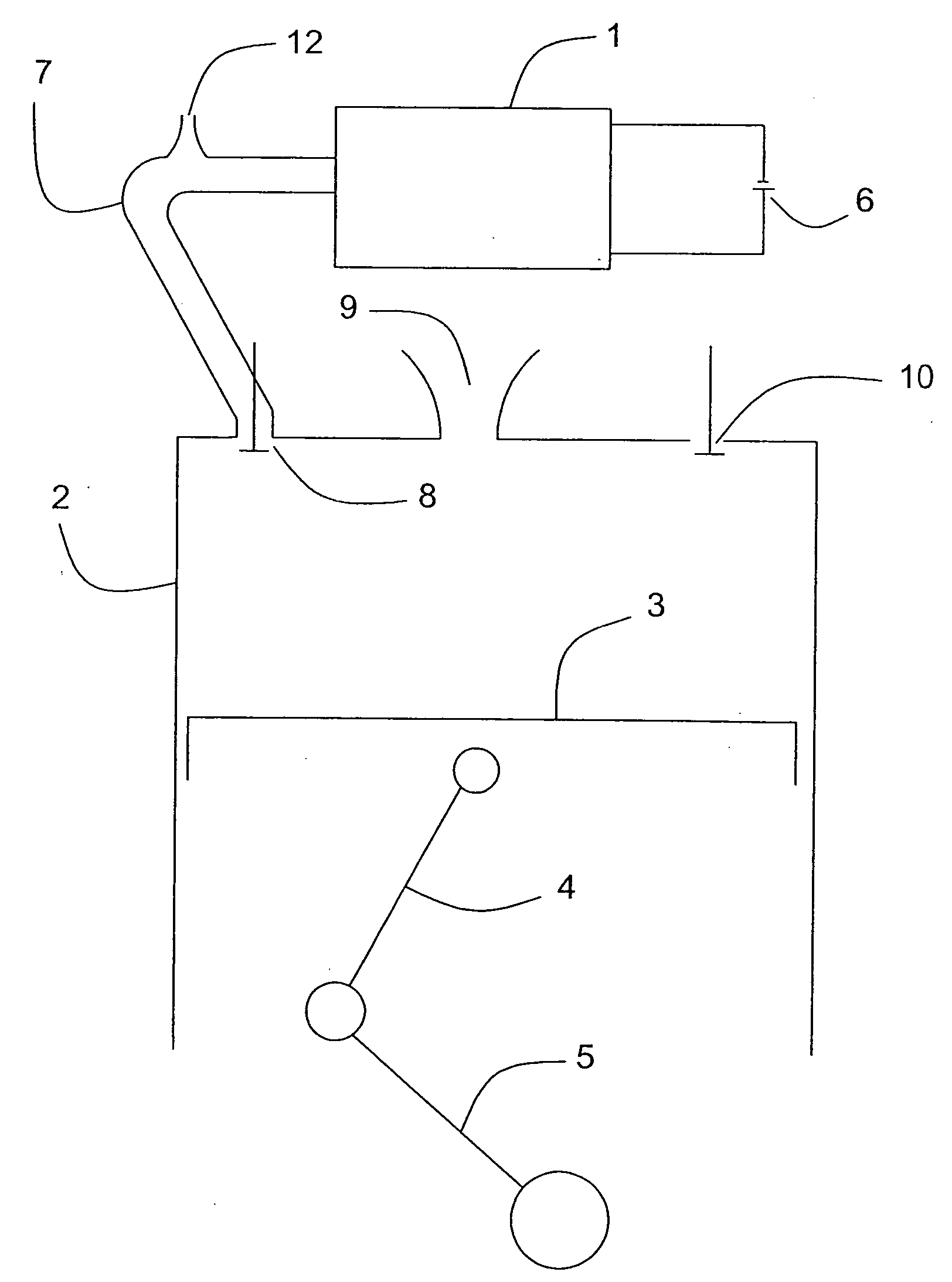
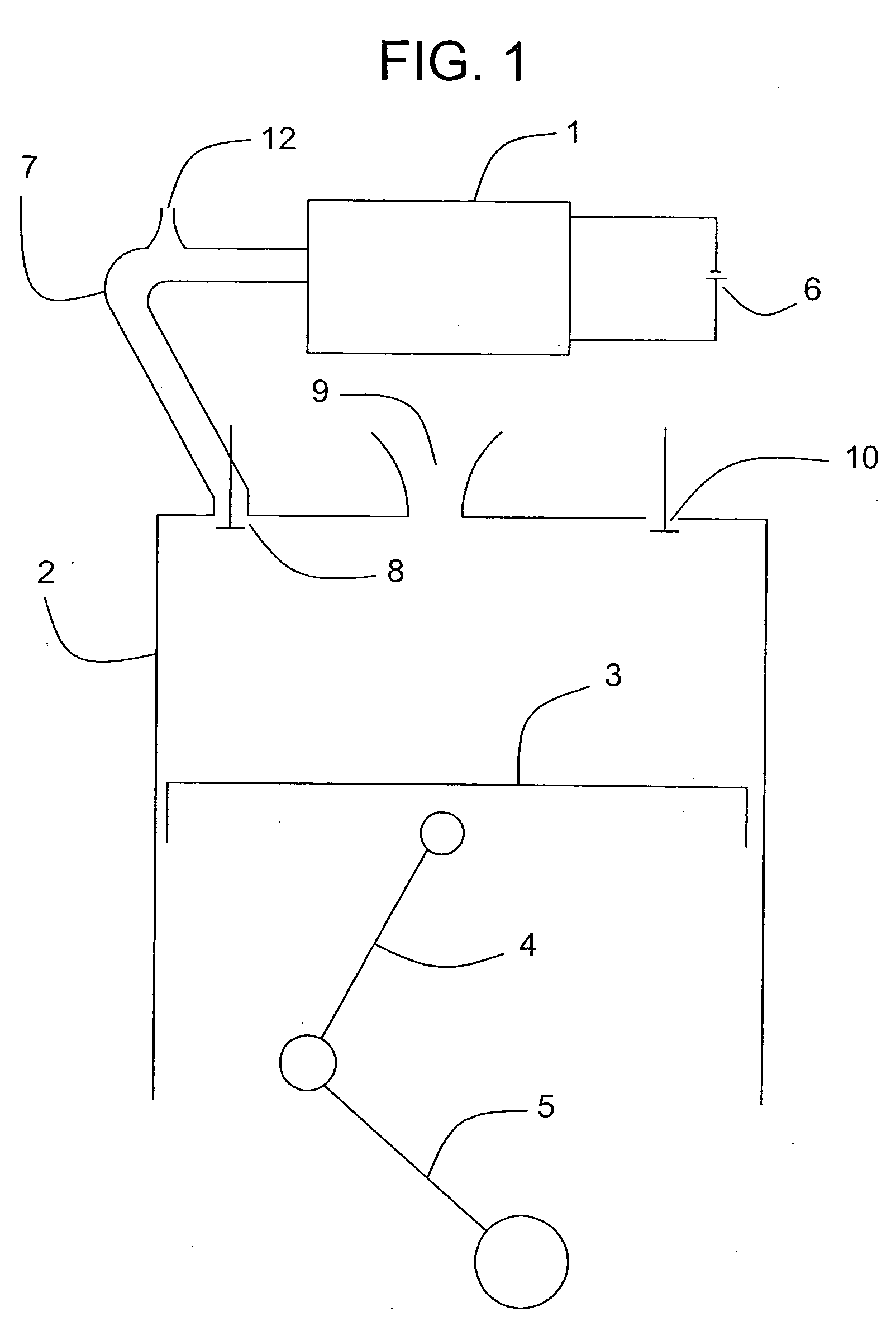
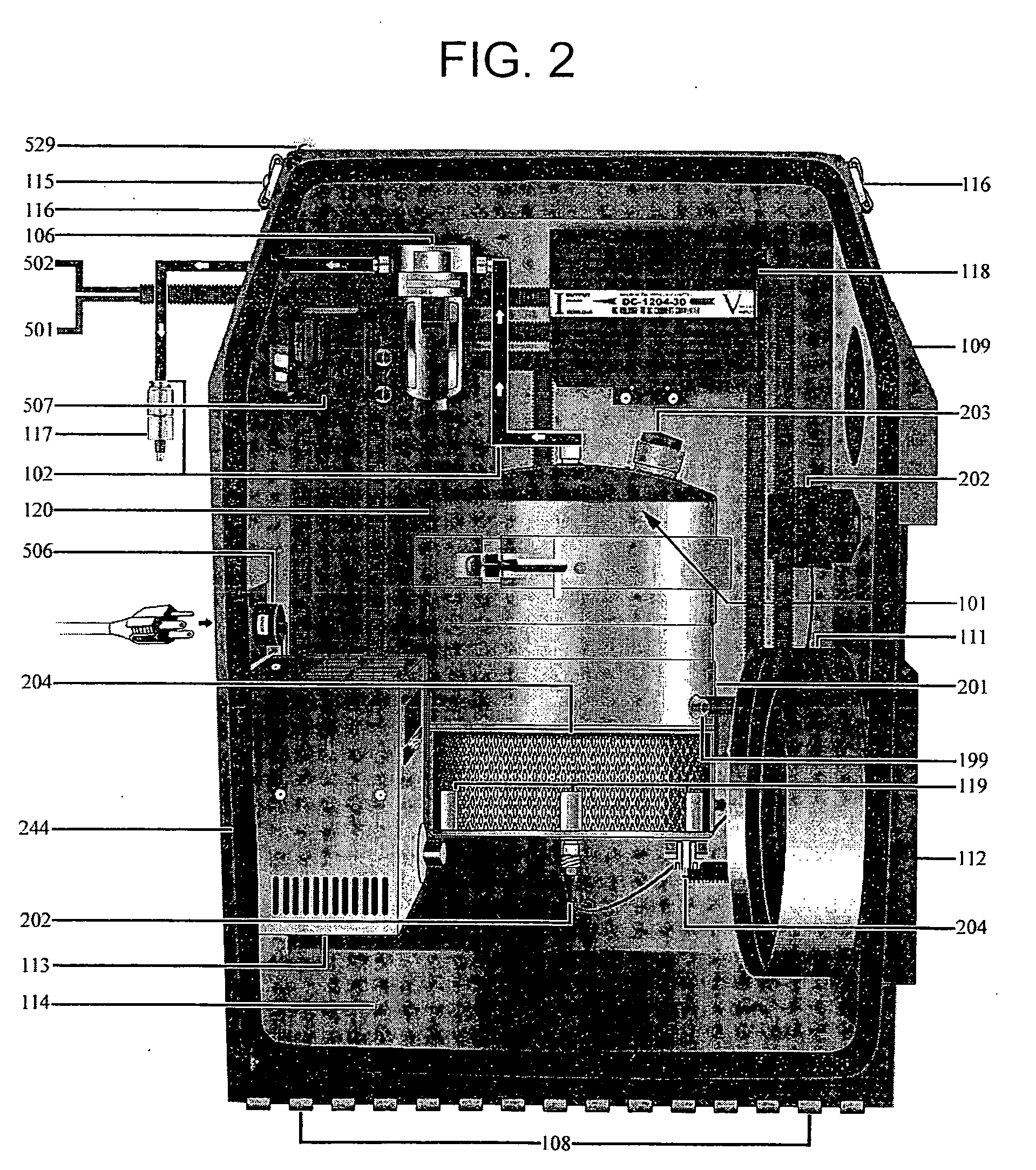
Internal combustion apparatus and method utilizing electrolysis cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089] The following experimental data shows an embodiment of the present disclosure operating with an increased efficiency, a lower NOx emissions while simultaneously not increasing NO2 emissions. The experimental data also illustrates a comparison of mileage increases between the present disclosures' operation and the hydrogen / oxygen fuel cell data disclosed and published in the Stowe Patent. The electrolysis cell used in the following experiments was based on a coated anode system (commercially available from CerAnode) having a mixed oxide coating believed to comprise a dual rutile phase of tantalum oxide and iridium oxide applied to a substrate comprising titanium alloy with less than 0.2 wt % palladium. These tests show some advantages that may be obtained under some conditions. Obviously, results may vary depending on a multitude of conditions including the condition of the engine, environmental conditions, fuel being used, etc. such that improvements are not seen in every ins...
example 2
[0093] The following emission tests were conducted using the same 2006 Dodge Ram 3500 with an 8 cylinder, 5.9L HO Cummins Turbo Diesel engine, 4-speed automatic transmission, 136-ampere alternator, 750-ampere battery, and 35 gal capacity fuel tank as used in the previously discussed testing. Baseline emissions readings were taken as an overall average for three tests based on each test using a five minute sampling period with a commercially available ECOM-AC Portable Emissions Analyzer. Emissions reading with the electrolysis cell of Example 1 operating were taken over extended thirty minute time periods in order to ensure that the cell had sufficient time to reach steady state conditions. Regardless of sampling time, each sampling event was conducted with the engine idling for about 1 hour at about 800 RPM (rotations per minute). The analyzer measured gases and calculated in PPM (parts per million) combustion parameters. The AC incorporated a high flow pump, a radiant gas cooler an...
example 3
[0095] This example demonstrates how various coated and uncoated anodes were evaluated to determine suitable anodes for long term use in potassium hydroxide electrolyte solutions in order to find materials that would have sufficient longevity of a vehicle, or approximately five to ten years. Accordingly, conventional accelerated testing conditions were determined based on using slightly concentrated potassium hydroxide at temperatures slightly above ambient and under electrolysis conditions of slightly increased current application.
[0096] Materials tested included 316 L stainless steel, 304 stainless steel, and 400 stainless steel, all of which disassociated producing rust resulting in contamination when used with any strength potassium hydroxide solution. Titanium metal reacted to reduce its conductivity when connected as an anode in any strength of potassium hydroxide. Nickel plate corroded, dissolved and left an undesirable black electrolyte. Copper metal turned green when used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com