Antibody complexes and methods for immunolabeling
an antibody complex and immunolabeling technology, applied in the field of immunolabeling complexes, can solve the problem that the time required for the labeling reagent to selectively bind to the target-binding antibody is typically very short, than 10 minutes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Fc Antigen
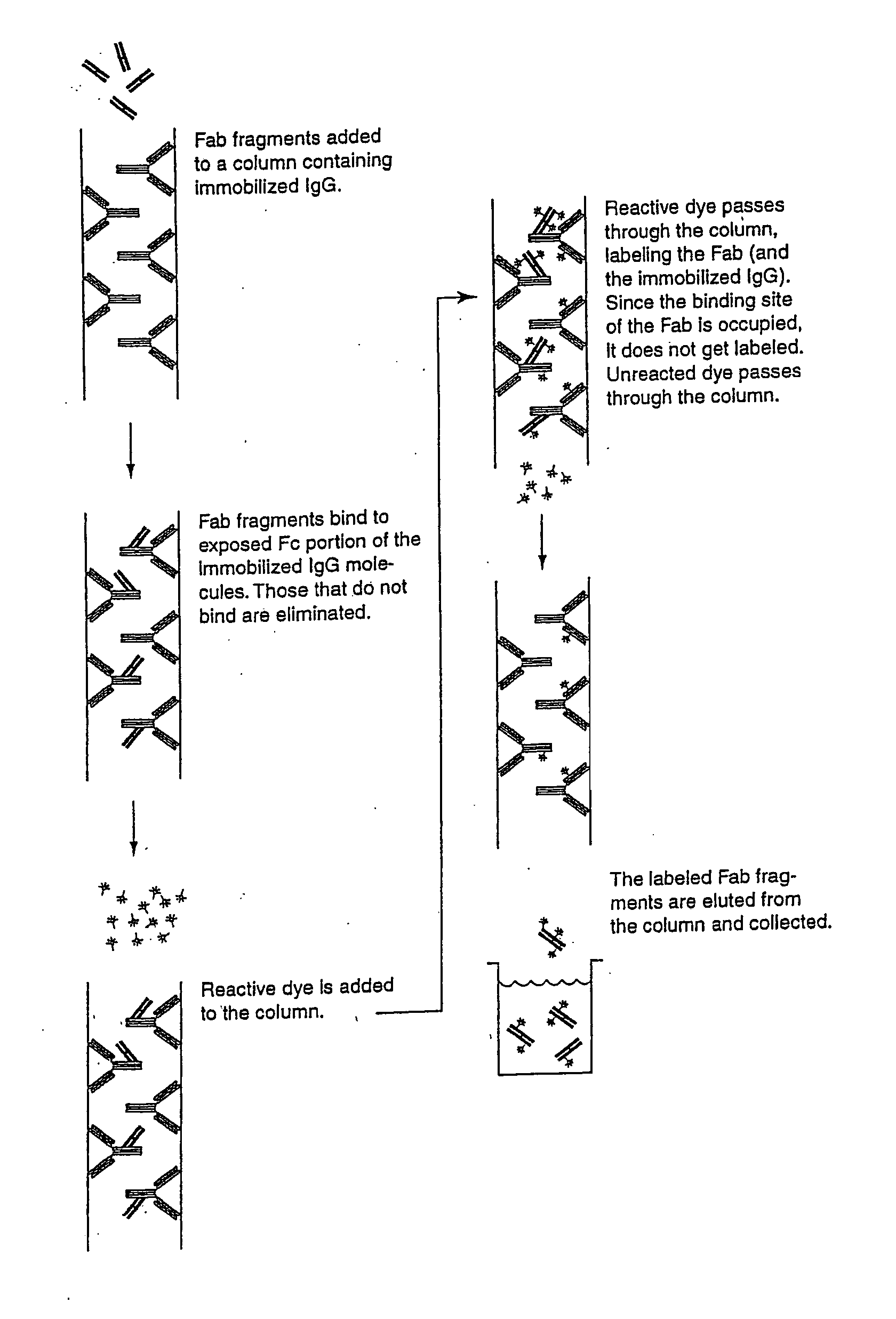
[0245] Purified mouse and rabbit IgG was fragmented with the proteolytic enzyme papain (CURRENT PROTOCOLS IN CELL BIOLOGY, 16.4.1-16.4.10 (2000)). A 12 mL solution of mouse IgG was prepared at ˜2 mg / mL in phosphate-buffered saline (PBS). A solution containing 0.1 mg of papain in digestion buffer (PBS, 0.02 M EDTA, 0.02 M cysteine) was added to the antibody and allowed to react at 37° C. for 16 hours. The digestion was terminated by the addition 20 μL of 0.3 M iodoacetamide in PBS. The fragments were dialyzed against 2 L of PBS for 16 hours at 4° C. The Fc fragment was purified on a protein G-Sepharose CL-4B column. The bound fraction containing the Fc fragment was eluted from the column using 50-100 mM glycine / HCl buffer, pH 2.5-2.8. The eluate was collected in 1 mL fractions. The pH of the protein fractions was immediately raised to neutral by addition of 100 μL of either 500 mM phosphate or Tris buffer, pH 7.6, to each 1 mL fraction. The solution was then...
example 2
Production of Anti-Fc Antibodies
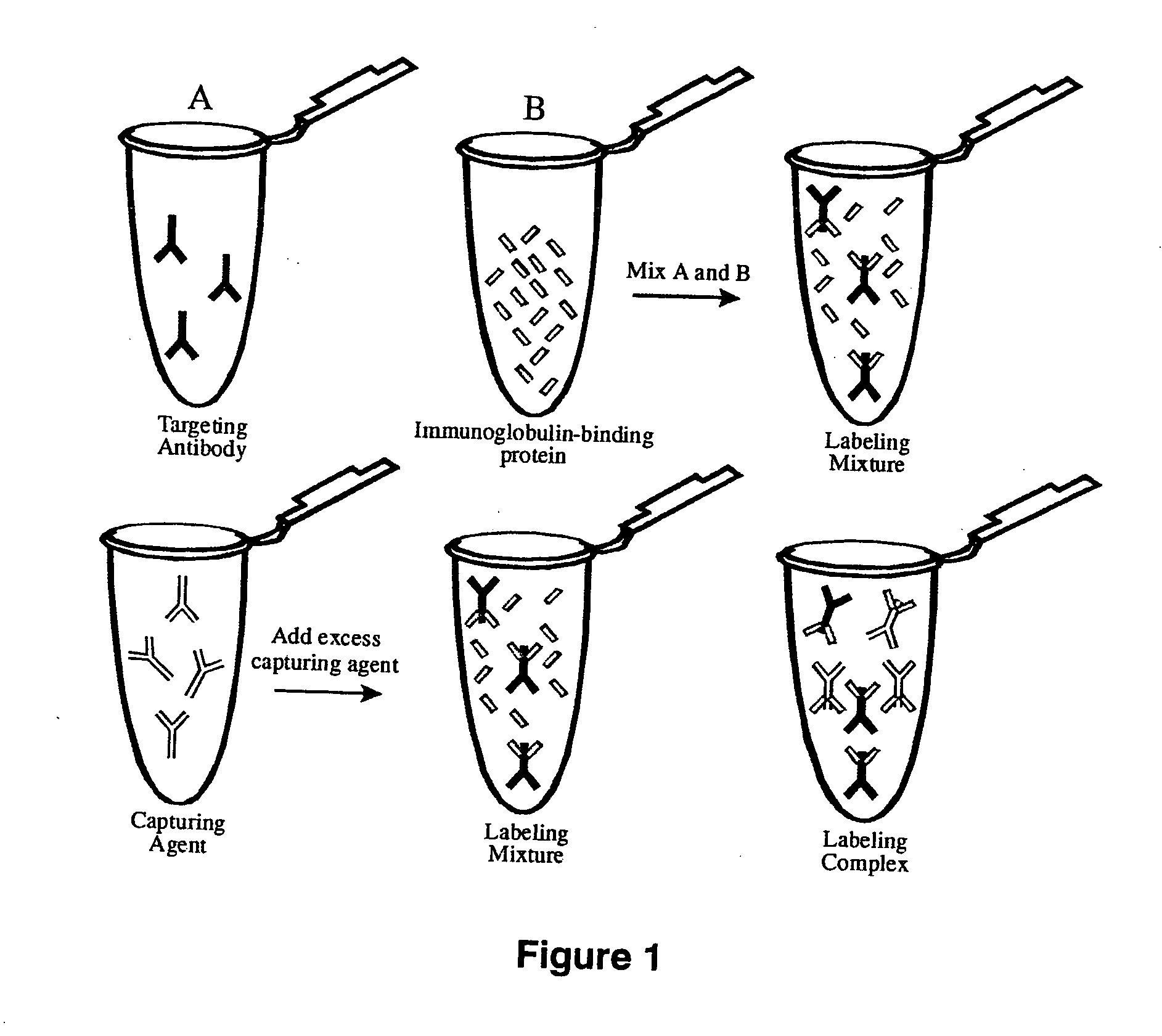
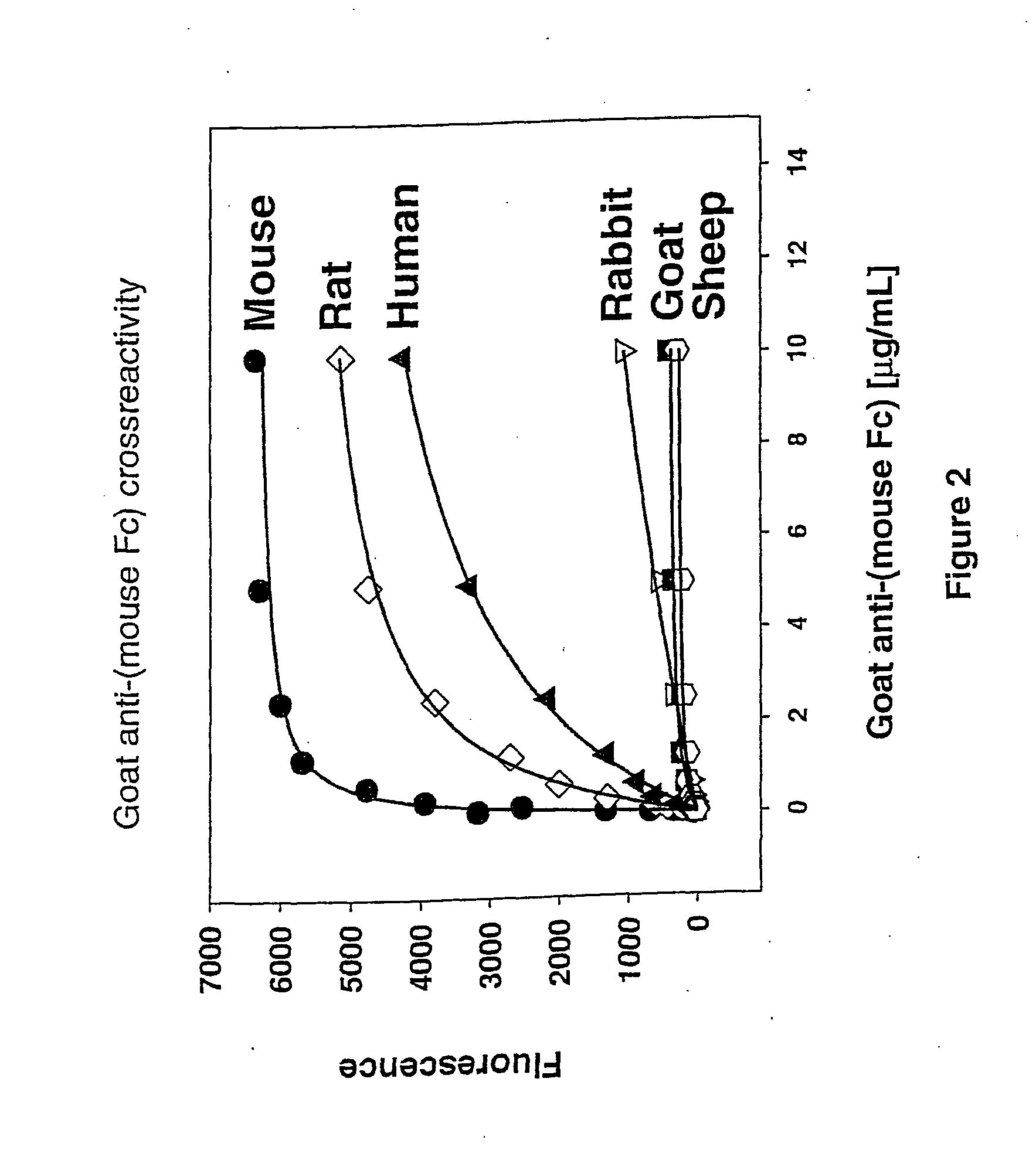
[0246] Polyclonal antibodies specific for the Fc region of an antibody were raised in goats against the purified FC region of an antibody from a different species (Example 1). Methods of immunizing animals are well known in the art, and suitable immunization protocols and immunogen concentrations can be readily determined by those skilled in the art (Current Protocols in Immunology 2.4.1-9 (1995); ILAR Journal 37, 93 (1995)). Briefly, individual goats were immunized with purified mouse Fc or purified rabbit Fc fragments. The initial immunization in 50% Freund's complete adjuvant (1000 μg conjugate (half subcutaneous, half intramuscularly)) was followed by 500 μg conjugate per goat in Freund's incomplete adjuvant two and four weeks later and at monthly intervals thereafter. Antibodies were purified from serum using protein A-Sepharose chromatography. Antibodies against mouse Fc isotypes can be prepared by starting with isotype-selected mouse Fc antige...
example 3
Preparation of Fab Fragments
[0247] Fragmentation of the goat anti-(mouse Fc) antibody to the monovalent Fab fragment was carried out using the proteolytic enzyme, papain, as described in Example 1. Following dialysis against PBS, the Fab fragment was purified on a protein A-Sepharose CL-4B column. The unbound fraction containing the Fab fragment and the papain was collected. This solution was then loaded onto a Sephacryl S-200 Superfine size-exclusion column and fractions corresponding to a molecular weight of ˜50 kDa were collected and analyzed by SDS-PAGE. The Fab fragments of goat anti-(rabbit Fc) can be prepared similarly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Color | aaaaa | aaaaa |
| Ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com