(Pentaphenyl)phenyl Group Containing Compound, Polymeric Derivative Thereof And Method For Forming The Same
a technology of pentaphenyl and compound, applied in the field of compound containing pentaphenyl phenyl group, can solve the problems of shortened lifetime, low glass transition temperature, brightness decay, etc., and achieve the effects of improving the optical and electrical properties of polymers, reducing molecular symmetry, and increasing the steric effect of polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0028]Referring to scheme 1, 5-bromo-2-iodo-pyridine as a starting substance with selective reactivity reacts with Pd(PPh3)4 organic metallic catalyst. At first, the Suzuki coupling reaction between the starting substance and boric acid with different electron-withdrawing group is carried out. Then, the Sonogashira coupling reaction with phenylacetylene is carried out to substitute the bromine atom at the fifth position of the starting substance. Thus, this method can successfully obtain compounds 4˜6 and have ideal yield.
[0029]Referring to scheme 2, the Diels-Alder reaction between the compounds 4˜6 and tetraphenylcyclopentadienone (TPCDO) is carried out. The reaction requires high temperature, about 260° C., and TPCDO is the reaction medium. Thus, no solvent is added. The reaction takes place about 3 hrs to obtain compounds 7˜9 containing a (pentaphenyl)phenyl substituent.
[0030]Referring to scheme 3, in the synthesis of the iridium complex, the method reported by Nonoyama is used ...
example 2
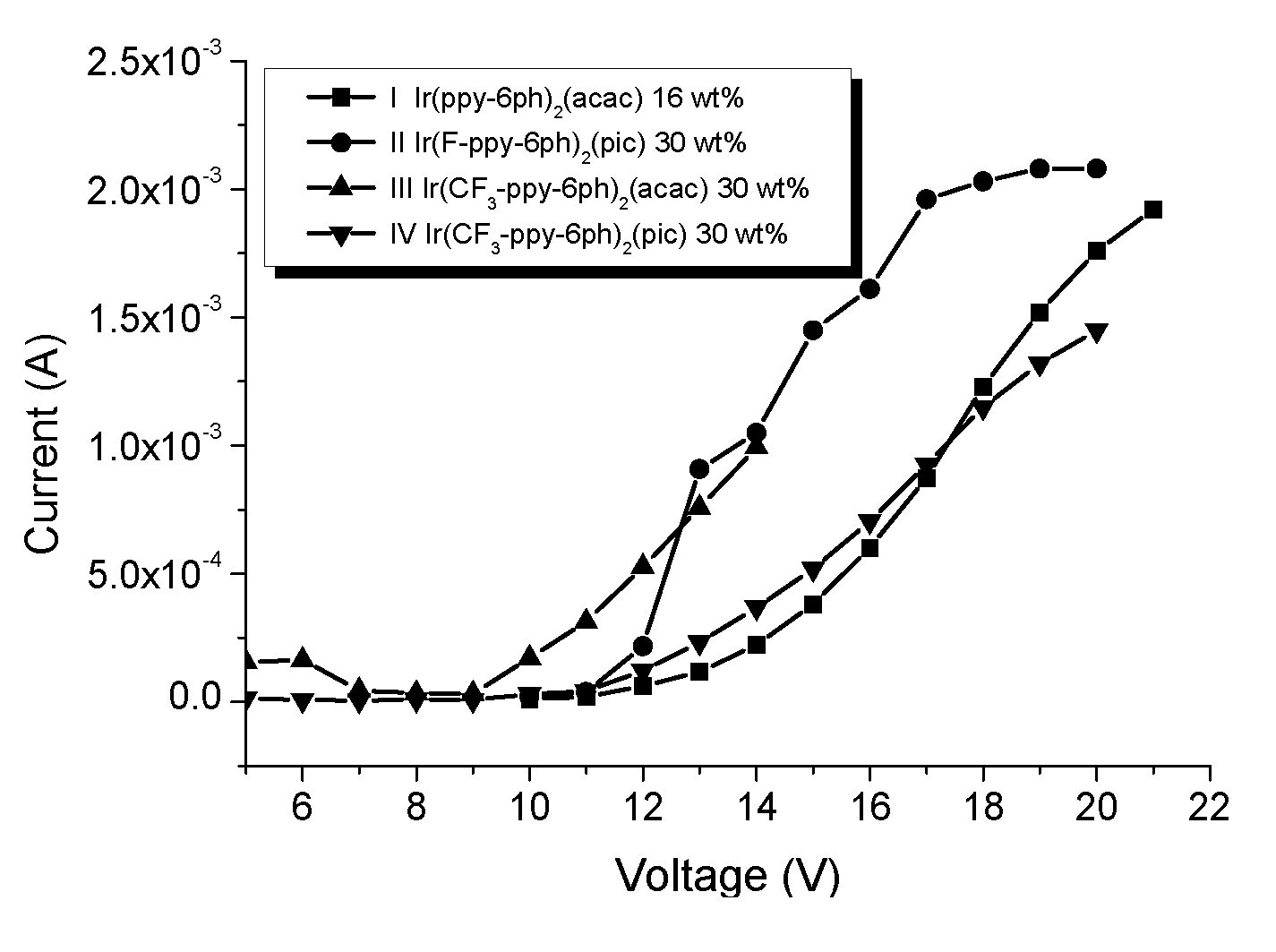
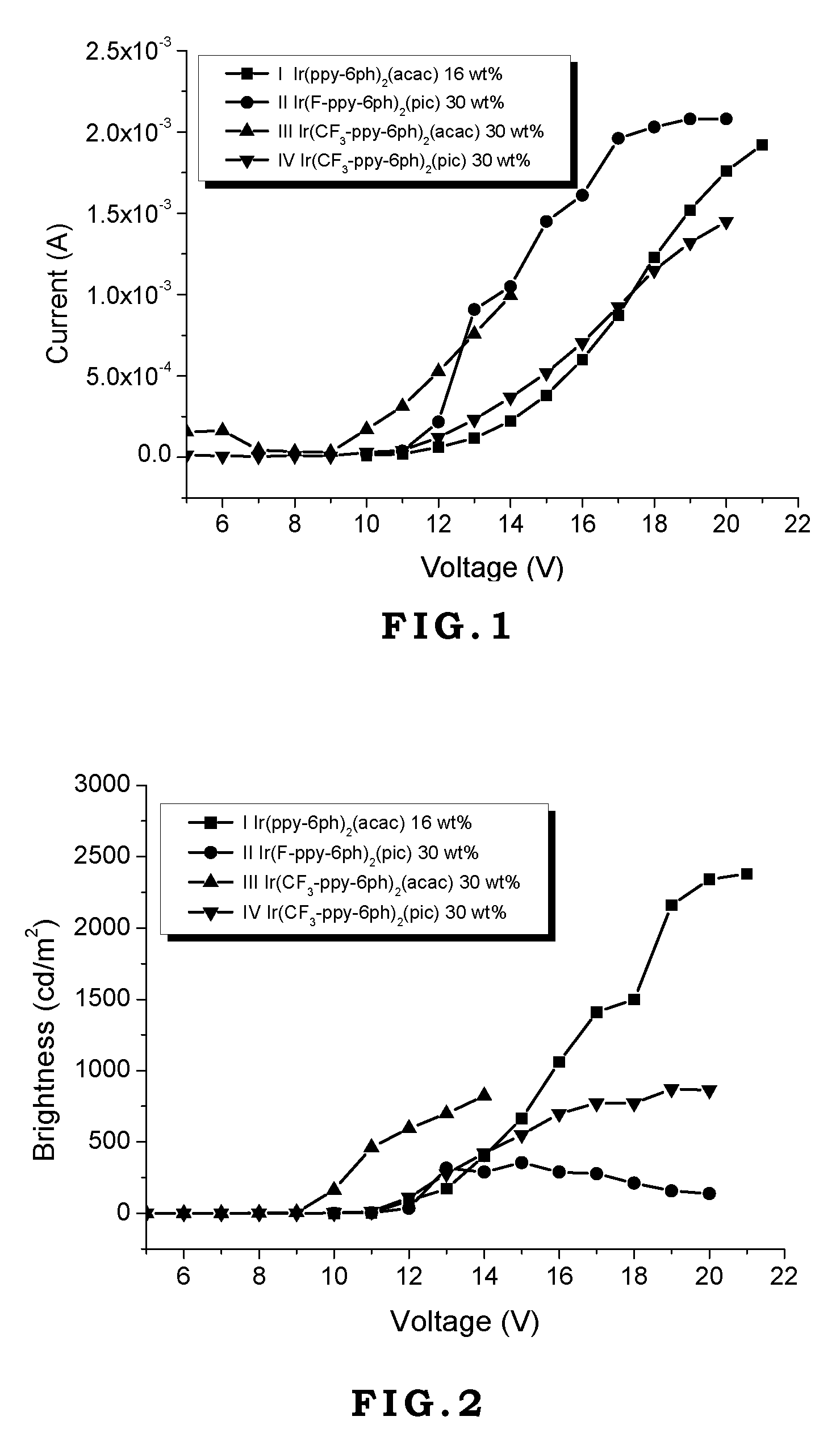
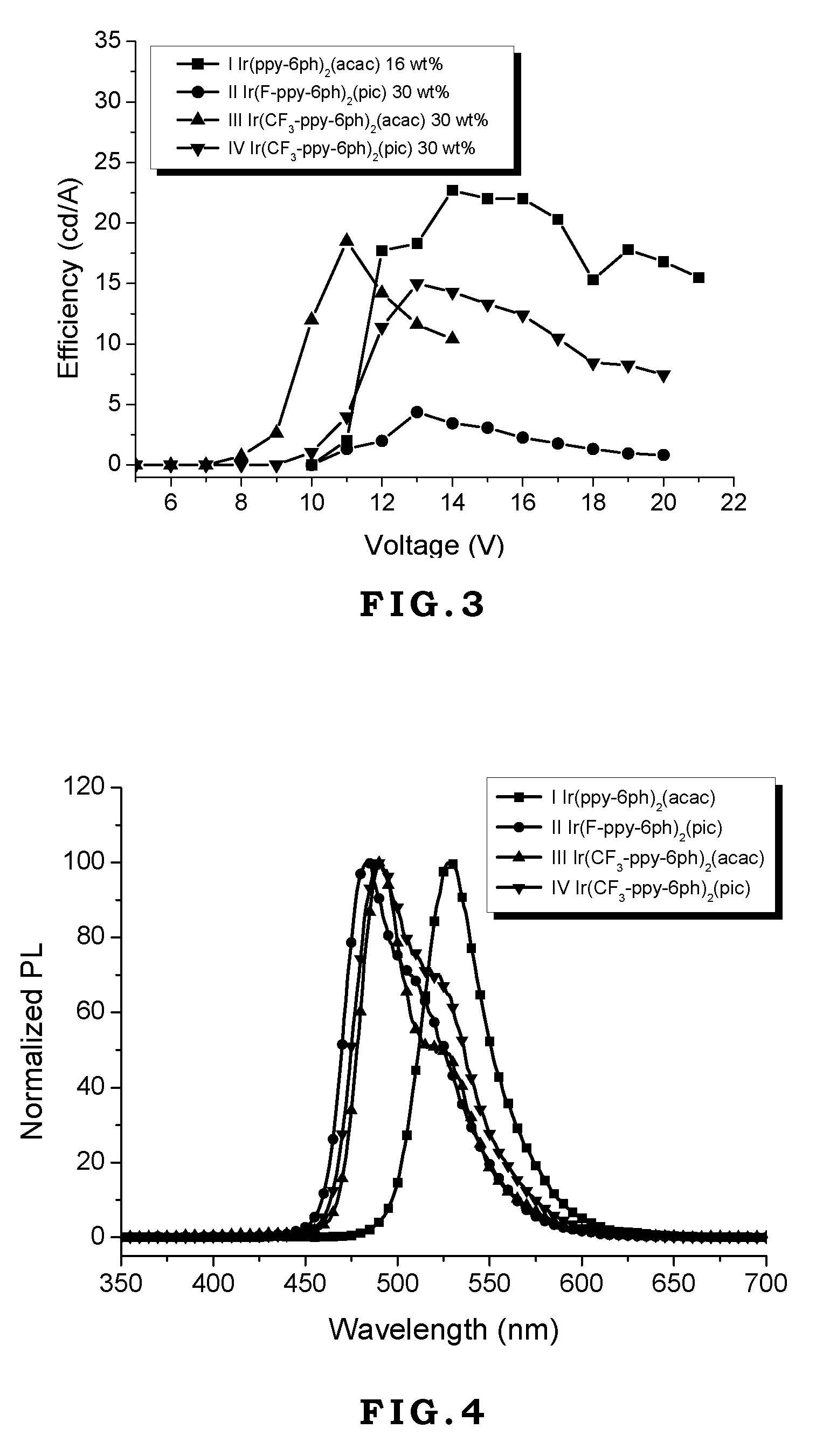
[0042]Generally, vacuum evaporation is utilized to fabricate the OLED devices. In the vacuum evaporation method, compounds are evaporated under vacuum and deposited on the ITO glass. However, the compounds have to sustain high temperature like 300˜400° C. without decomposition or transformation. If the compounds decompose during the evaporation process, the decomposed compounds will contaminate the device to jeopardize the performance of the device. In the present invention, although the device is made by spin coating without direct heating compounds, the external current applied on these compounds during operating or testing generates heat and transfers heat to these compounds. Therefore, the materials used in the OLED devices require thermal stability. If the thermal decomposition temperature (Td) of the material is too low, its application will be limited.
[0043]On the other hand, the glass transition temperature (Tg) of the material also affects the lifetime, efficiency, and chro...
example 4
[0051]This example provides seven polymers derived from a 9-[(pentaphenyl)phenyl]carbazole structure, as shown in the following P1-P7, and a comparison polymer P8. The synthesis of the polymers P1, P2, and P4-P8 utilizes Suzuki coupling reaction. At first, borate or boric acid compound monomer and brominated monomer are dissolved in THF (as the solvent), Pd(PPh3)4 is the catalyst, P(t-Bu)3 is the ligand, K3PO4 is also added, and reflux reaction is carried out for 40 hrs under the reaction temperature of 70° C. Next, triphenylammonium bromide as a terminal group is added and the reaction proceeds for one additional day and then stops. Finally, chloroform is used to extract the crude polymer product. Toluene is used as the elute and the chromatographic column is used to remove salts and heavy metal catalyst. The product is treated by vacuum concentration and then reprecipitated in methanol. Thus, the purified polymer is obtained. The molecular weights and distribution of the polymers ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com