Construction of Arterial Occlusive Disease Animal Model
a construction and occlusive disease technology, applied in educational models, medical science, diagnostics, etc., can solve the problems of high mortality within a relatively short period, lack of stable pathologic conditions, and high probability of death, and achieve stable pathologic conditions, low animal mortality, and high certainty of vascular embolism.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of a Pig Model of Chronic Cardiac Infarction
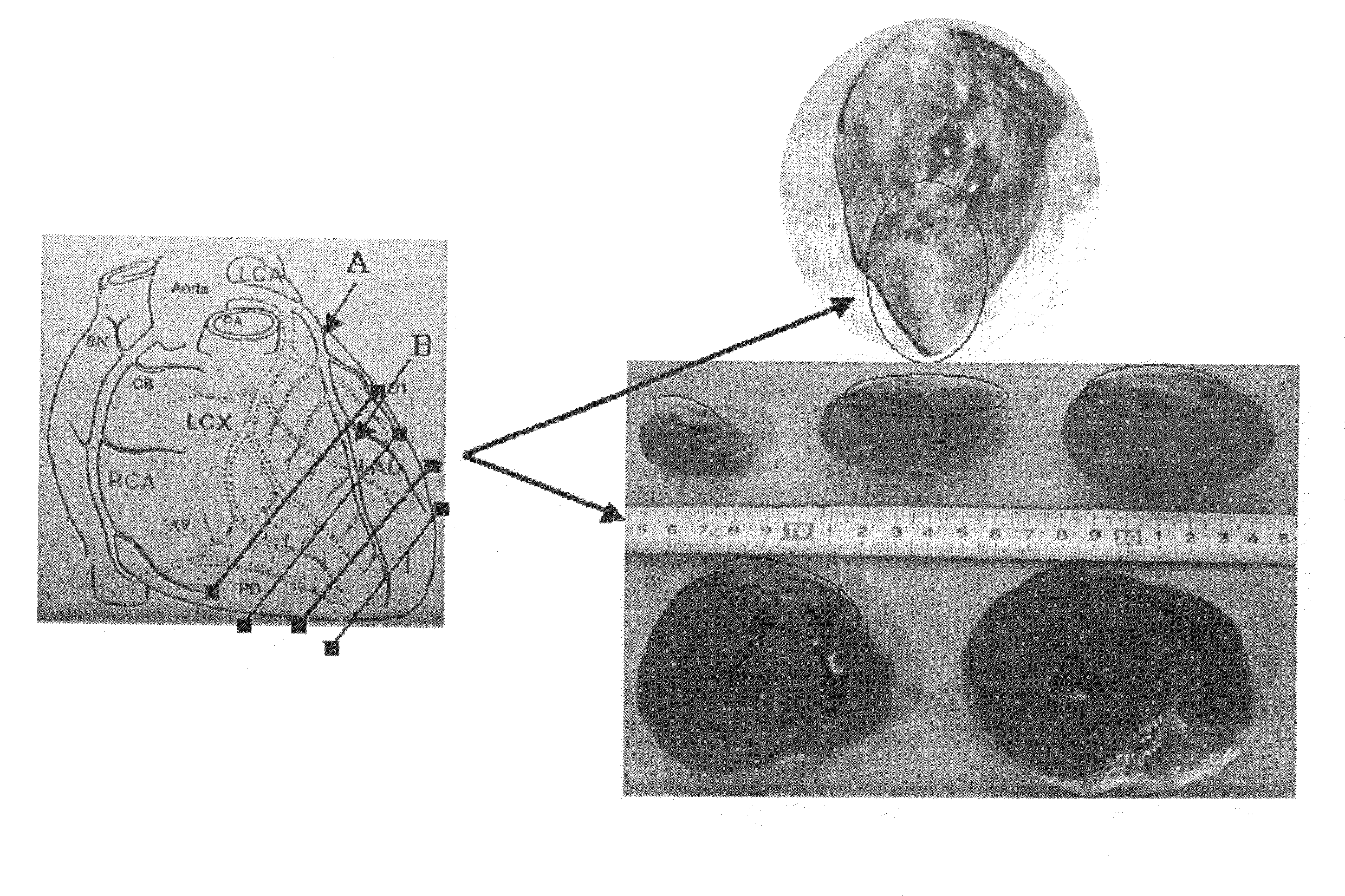
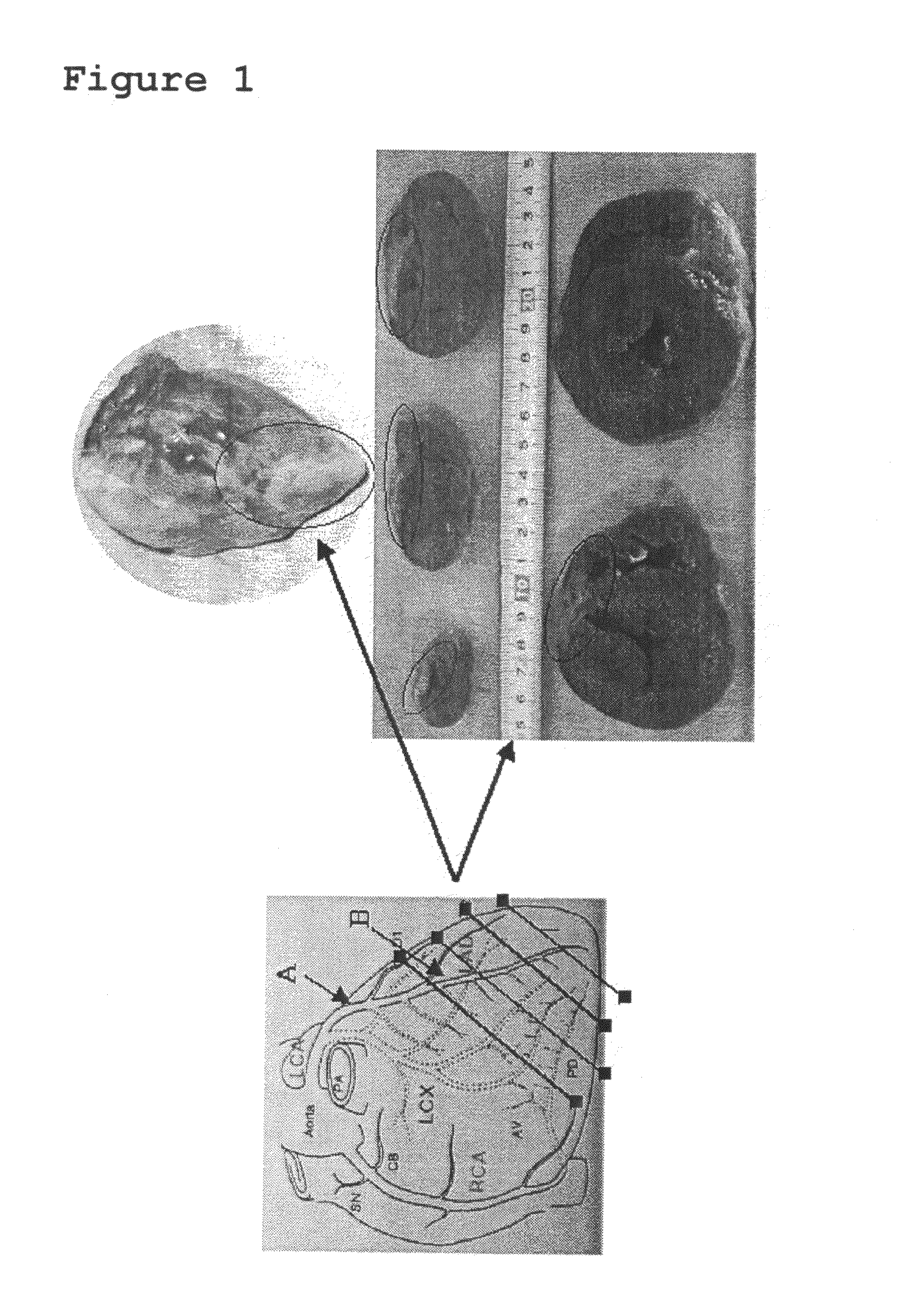
[0040]Male pigs weighing 20-25 kg were used. After anesthesia, pigs were fixed in the recumbent position with the left chest up and an anticoagulant, heparin, was administered at 100 IU / kg, followed by thoracotomy by dissection between the third and fourth ribs. Subsequently, portion beyond the second branch of the anterior descending branch was exposed and completely ligated with a suture (see arrow B of the left panel in FIG. 1). (Complete ligating at this site does not give rise to fibrillation, leading to no death. This local ischemic treatment results in the tolerance to ischemia in the entire cardiac muscle.) After that, portion of the anterior descending branch which was proximate to the bifurcation of the circumflex branch and the anterior descending branch was exposed and a xylocalne jelly was applied to the blood vessel to prevent vascular spasm associated with the treatment, followed by placement of an ameroid ring (a...
example 2
Studies on Coagulating Agents, Conditions, and Others
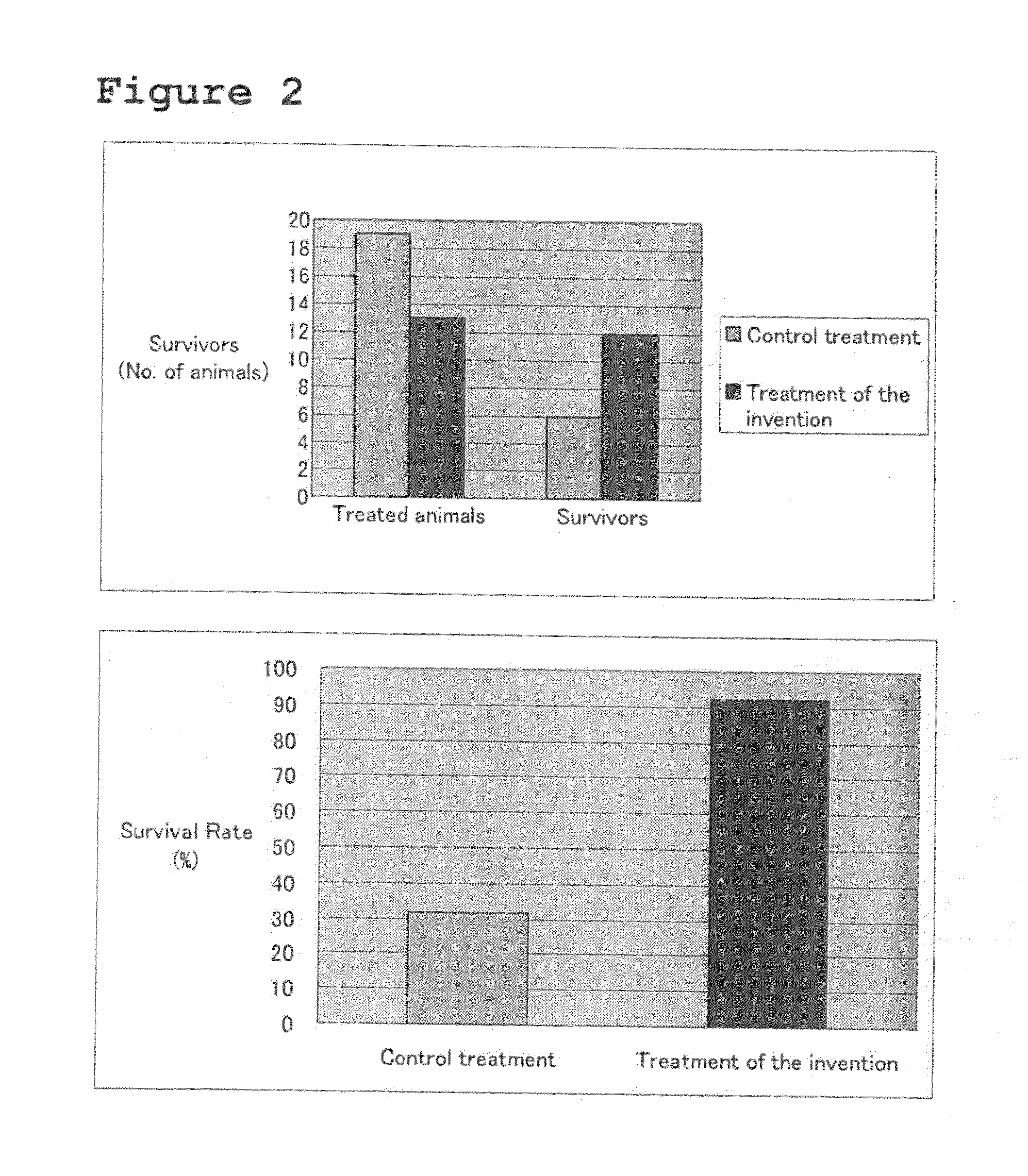
[0044]As coagulating agents, ADP and thrombin were used to examine the degree of coagulation. ADP was used at concentrations of 30 and 100 μg / ml (without thrombin at both concentrations), and thrombin at concentrations of 0.5 and 10% (w / v) (without ADP at both concentrations). Blood was drawn from the ventral tail artery of a crab-eating macaque, and was placed into a catheter with or without adding a coagulating agent, incubated for 24 hours at 37° C., and then pushed out to observe the condition of coagulation. As control was used a sample to which neither of the coagulating agents was added (blood which was coagulated alone). The results were shown in the left panel of FIG. 3. It was found that the higher concentration of ADP, the better coagulation took place. Thrombin resulted in comparable coagulation at both 0.5 and 10% (w / v). Next, a blood clot which was obtained by incubating blood for 24 hours at 37° C. with 100 μg of AD...
example 3
Generation of an Acute Cerebral Trunk Arterial Embolus Model
[0045]As a study animal was utilized a male crab-eating macaque weighing about 6 kg. A sufficient amount of blood (1 ml) was drawn from the ventral tail artery of the animal with a syringe containing in advance ADP (a final concentration of 100 μg / ml) and thrombin (a final concentration of 10% (w / v)) as coagulants and filled immediately into a polyethylene tube (O.D.: 0.965 mm, I.D.: 0.58 mm). An embolus having a well-solidified state was formed by leaving the mixture of the blood and coagulants at 37° C. for 24 hours while keeping the mixture in the tube. Subsequently, a very fine catheter for cerebral angiography which was in conformity to an arterial diameter (3 French) was used and inserted through a sheath placed into femoral artery, so as to place the catheter in a targeted cerebral vessel (left middle cerebral artery). During that, the catheter was guided by using a high-resolution X-ray cerebral angiographic apparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com