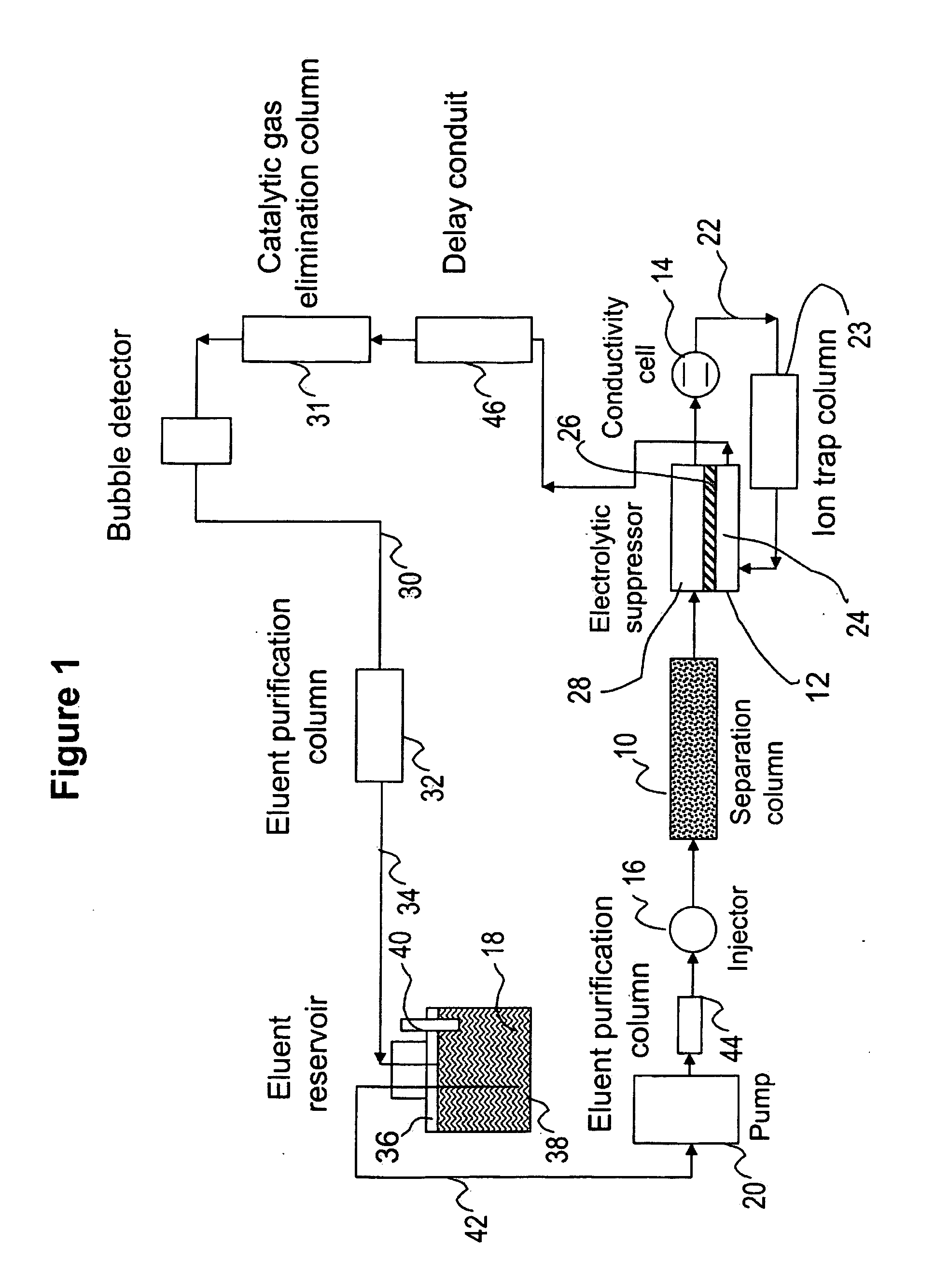
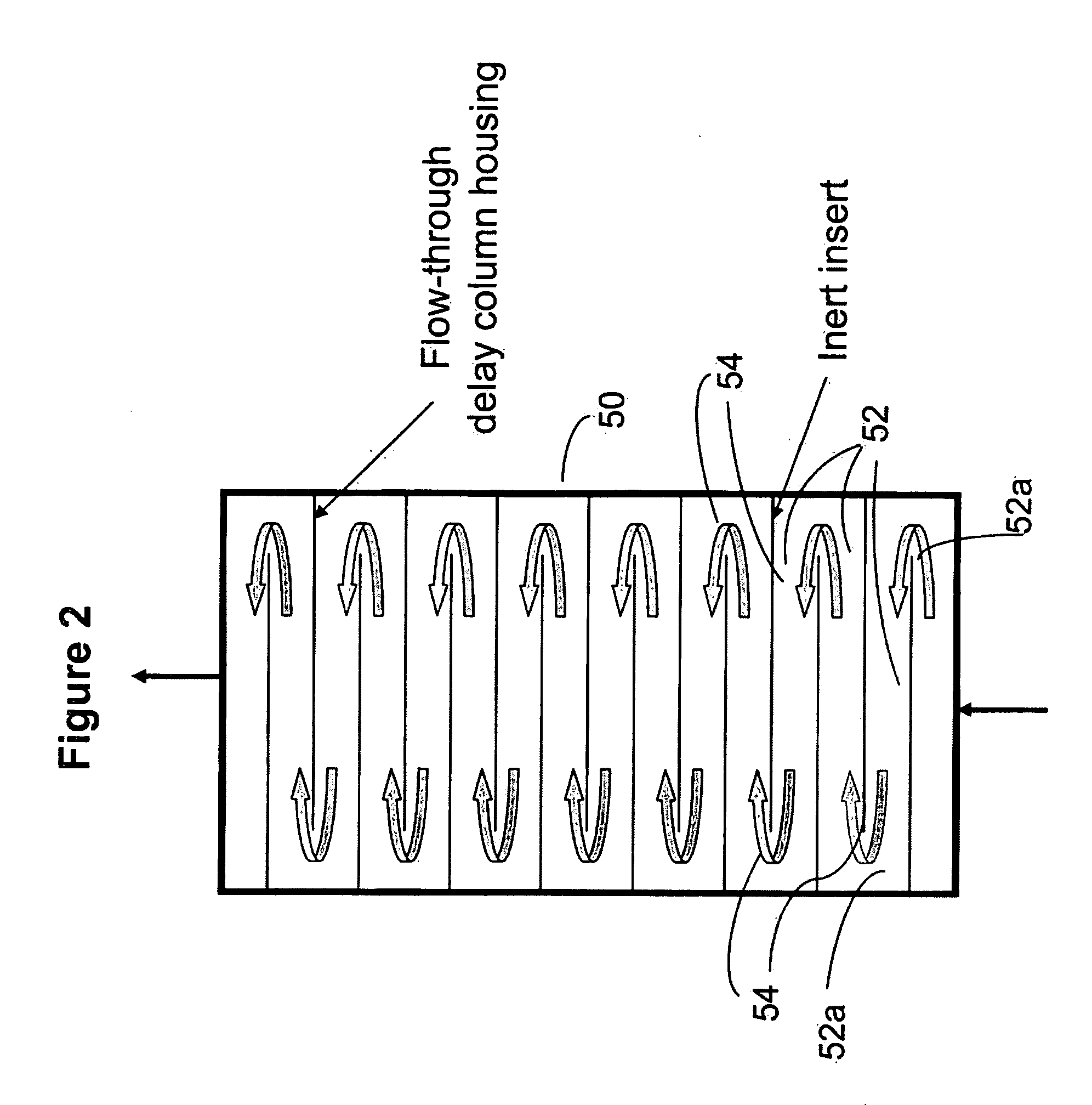
Ion chromatography systems with flow-delay eluent recycle
a technology of chromatography and eluent recycling, which is applied in the field of ion chromatography, can solve the problems of easy contamination of carbonate, easy operator errors, and laborious off-line preparation of chromatographic eluents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0104]Ion chromatographic separation of common anions using an electrolytic suppressor and recycle of a sodium carbonate / sodium bicarbonate eluent.
[0105]This example illustrates the use of the eluent-recycle ion chromatography system shown in FIG. 1 for determination of common anions including fluoride, chloride, nitrate, phosphate and sulfate. A Dionex ICS-2000 ion chromatography system consisting of a dual-piston high pressure pump, a six-port injector, a column oven, and a conductivity detector was used. A Dionex 4-mm AS22 column (4 mm.×250 mm) was used as the separation column. A solution of 4.5 mM sodium carbonate and 1.2 mM sodium bicarbonate was used as the eluent, and the separation was performed at 1.2 mL / min. A Dionex ASRS-300 electrolytic suppressor was used in the experiments. A flow-through delay column (10 mm×250 mm) was used. The catalytic gas elimination column contains a cation exchange resin coated with Pt. The eluent purification columns (9 mm×85 mm) were packed w...
example 2
[0107]Ion chromatographic separation of common cations using an electrolytic suppressor and recycle of a methanesulfonic eluent.
[0108]This example illustrates the use of an ion chromatography system with eluent recycle shown in FIG. 1 for determination of common cations including lithium, sodium, ammonium, potassium, magnesium, and calcium. A Dionex ICS-2000 ion chromatography system consisting of a dual-piston high pressure pump, a six-port injector, a column oven, and a conductivity detector was used. A Dionex 4-mm CS12A column (4 mm×250 mm) was used as the separation column, a solution of 20 mN methanesulfonic acid was used as the eluent, and the separation was performed at 1.0 mL / min. A Dionex CSRS-300 electrolytic suppressor was used in the experiments. A flow-through delay column (10 mm×250 mm) was used. The catalytic gas elimination column contains a cation exchange resin coated with Pt. The eluent purification columns (9 mm×85 mm) were packed with appropriate ion exchange re...
example 3
[0110]This example describes a process of preparing a platinum coated resin for this application. A cation exchange resin (commercially available from various resin manufacturers such as Rohm and Haas; Dow Chemicals etc) was used as the substrate. 100 g of the resin was converted to the sodium form using 1 M sodium hydroxide. Next the resin was washed with DI water and filtered. The resin was added to a 2 L bottle and the appropriate catalyst solution (For example Tetraammineplatinum (II) Chloride solution) app. 630 ml at a concentration of 4000 mg / L was added. The bottle was capped and tumbled to mix the resin intimately with the catalyst solution for 2 hours. The resin was washed and filtered. At this point a layer of platinum is adhered to the resin by electrostatic means. Next, a 5% borohydride solution was made and the resin was placed in this container and mixed with care. The container is left open to ambient environment (due to bubbling in the container) and placed in an ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com