Biosensor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0071]Into a 500-mL Sakaguchi flask, 400 mL of culture media composed of sorbitol (2% by weight), yeast extract (0.3% by weight), meat extract (0.3% by weight), corn steep liquor (0.3% by weight), polypeptone (1% by weight), urea (0.1% by weight), KH2PO4 (0.1% by weight), MgSO4.7H2O (0.02% by weight), CaCl2 (0.1% by weight), with a pH of 7.0, was transferred, by 100 mL per one flask, and autoclaved at 121° C. for 20 minutes.
[0072]As an inoculum, a strain of Gluconobacter Oxydans NBRC 3291, was inoculated by one platinum loop and cultured at 30° C. for 24 hours to make a seed culture media.
[0073]Then, 6.6 L of the culture media prepared with the same composition as in the above, was transferred to a 10-L jar fermenter, and autoclaved at 121° C. for 20 minutes, followed by gradually cooling and subsequently transferring of a 400 mL of the seed culture media, which was cultured under 750 rpm and an airflow amount of 7 L / minute, at 30° C. for 24 hours.
[0074]The culture media was subject...
example 1-1
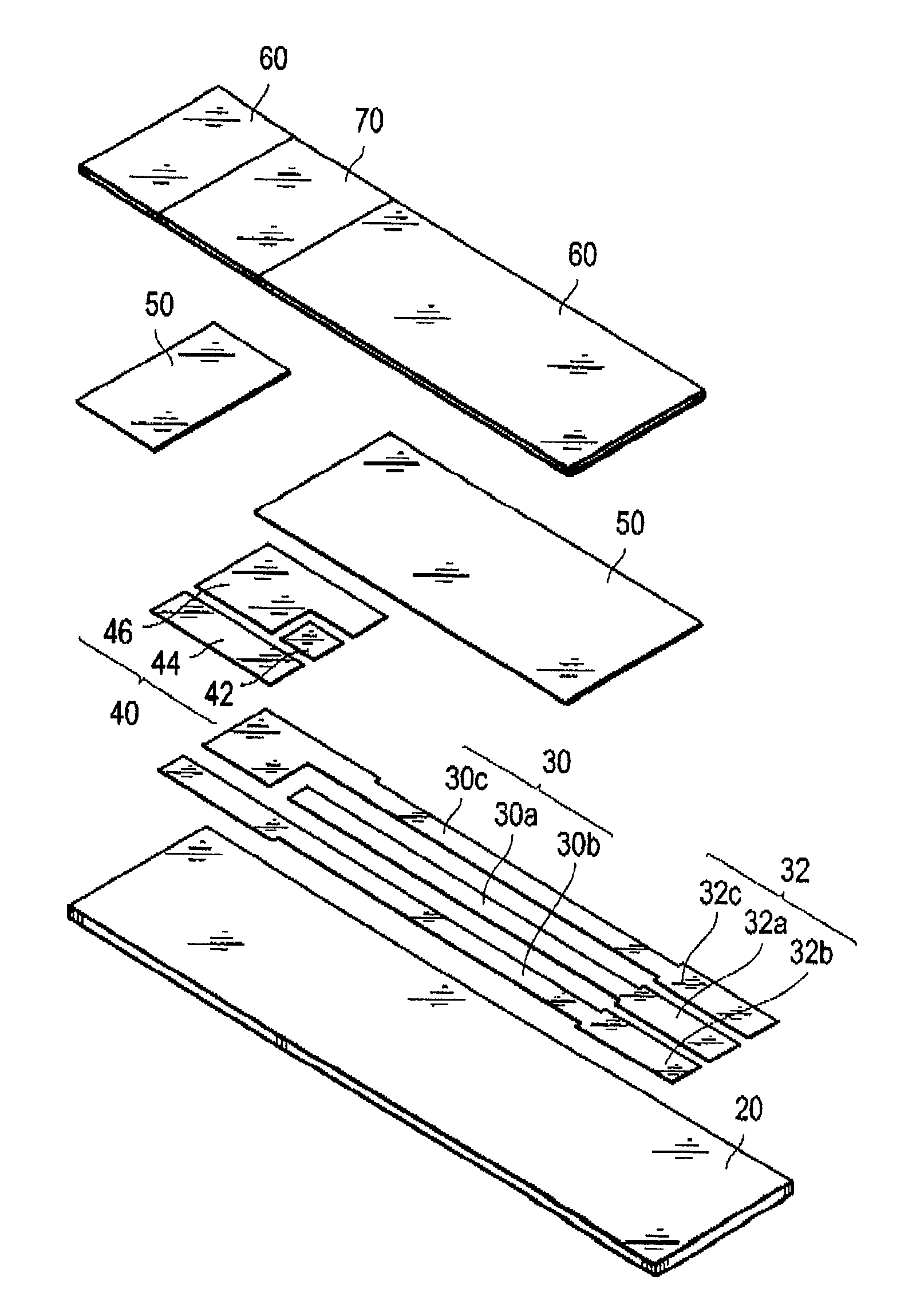
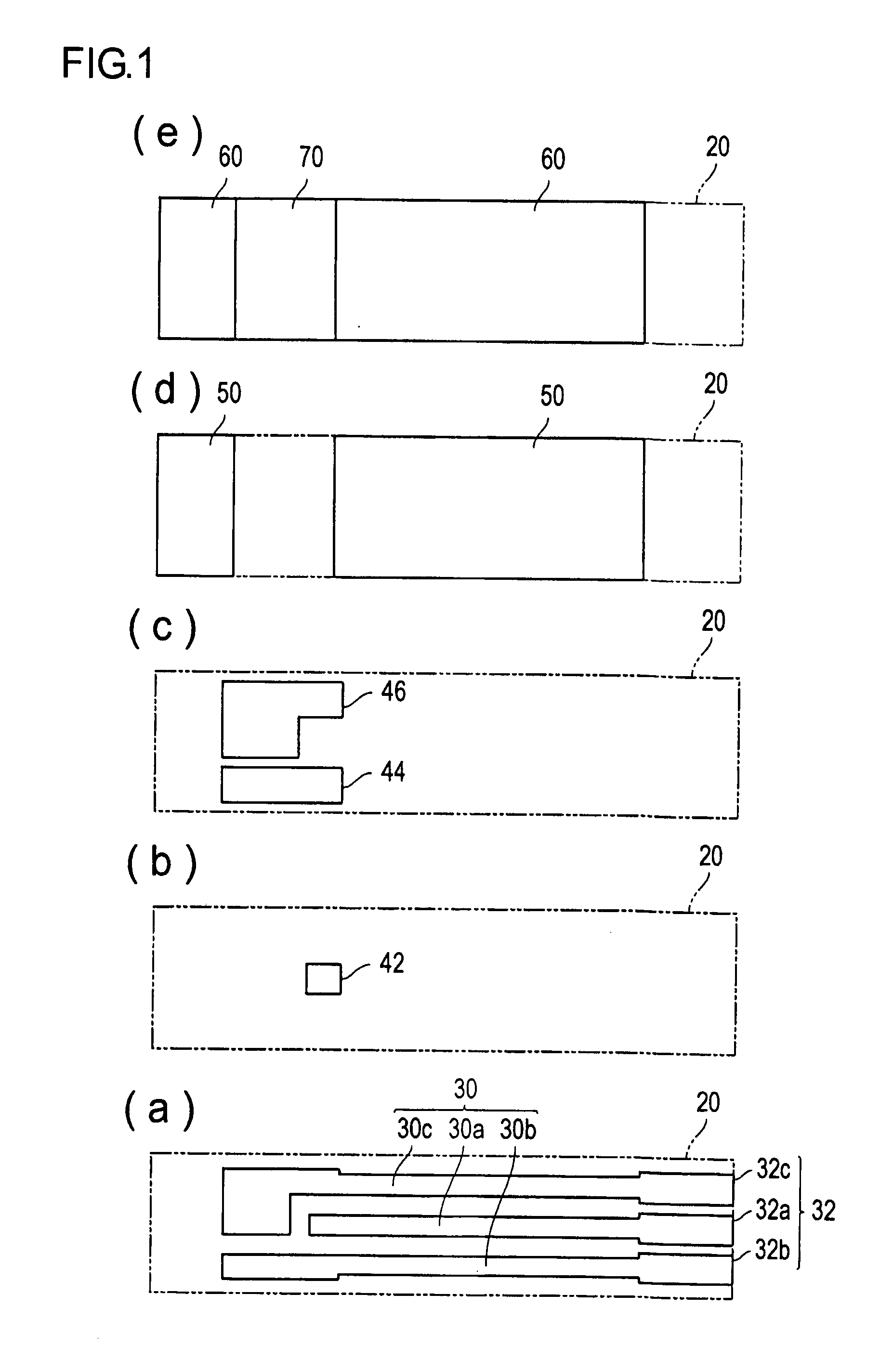
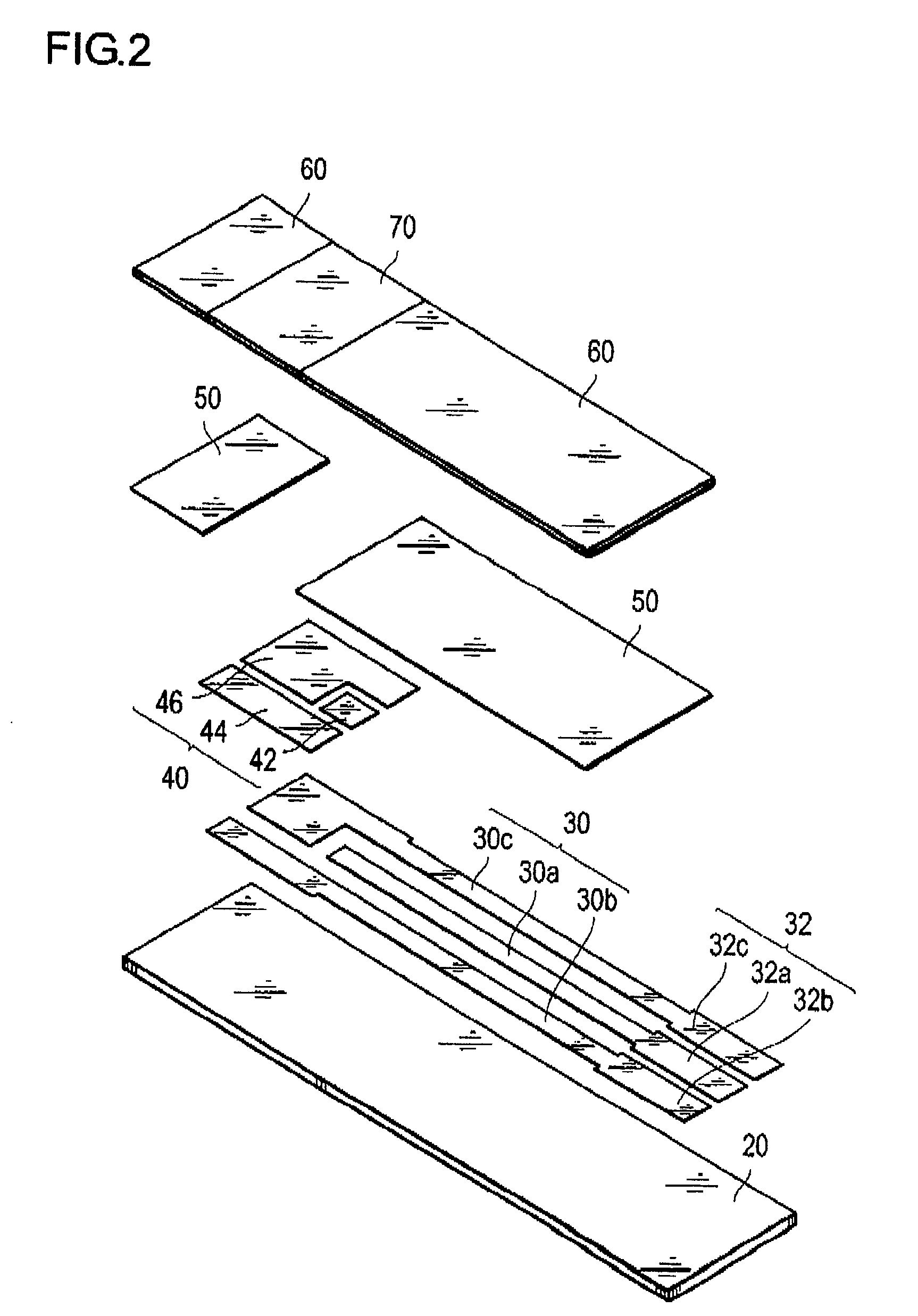
[0075]As a sensor substrate formed with an electrode system, a commercial sensor electrode (manufactured by BVT Co., Ltd; ACl.W5.R1) was prepared. In this sensor substrate, the electrode system has a diameter of about 6 mm, and nearly circular shape. Furthermore, at the upper part of the electrode system prepared above, a PET sheet, with a thickness of 0.2 mm, and having a hole with a diameter of 6 mm, was glued together to form a spacer. Into the inside of the hole of the spacer on the electrode system, 10 μL of phosphate buffered saline mixed with GlyDH (20 U / mL) obtained in the above Reference Example 1, the lipoprotein lipase (manufactured by Amano Enzyme Inc., 10,000 U / mL), and 1-methoxy-5-methylphenazinium methylsulfate (manufactured by DOJINDO Laboratories, 10 mmol / L; hereinafter referred to as “m-PMS”) as an electron mediator, was dropped, and dried at room temperature, to form a reaction layer.
[0076]Onto this reaction layer, 10 μL of a sample liquid containing 50 mg / dL of t...
example 1-2
[0081]After dropping an aqueous solution of 0.5% (w / v) of sodium carboxymethyl cellulose (manufactured by Wako Pure Chemical Industries Ltd; hereafter may be referred to as “CMC”) onto the electrode system used in Example 1-1, and drying, a 0.5% CMC solution containing GlyDH (20 U / mL) obtained similarly as in Example 1-1, the lipoprotein lipase (10,000 u / mL) and m-PMS (10 mmol / L) was dropped, and dried to form a reaction layer.
[0082]Correlativity between response current value and triolein concentration was studied similarly as in Example 1-1, by using the biosensor. The result is shown in FIG. 5.
[0083]As shown in FIG. 5, although the resultant response current value was reduced a little by effect of CMC, higher correlativity between response current value and concentration of neutral fat was observed as compared with Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com