Method for Detecting Transferase Enzymatic Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Determining the Activity of Lipid Dependent Serine / Threonine Kinases
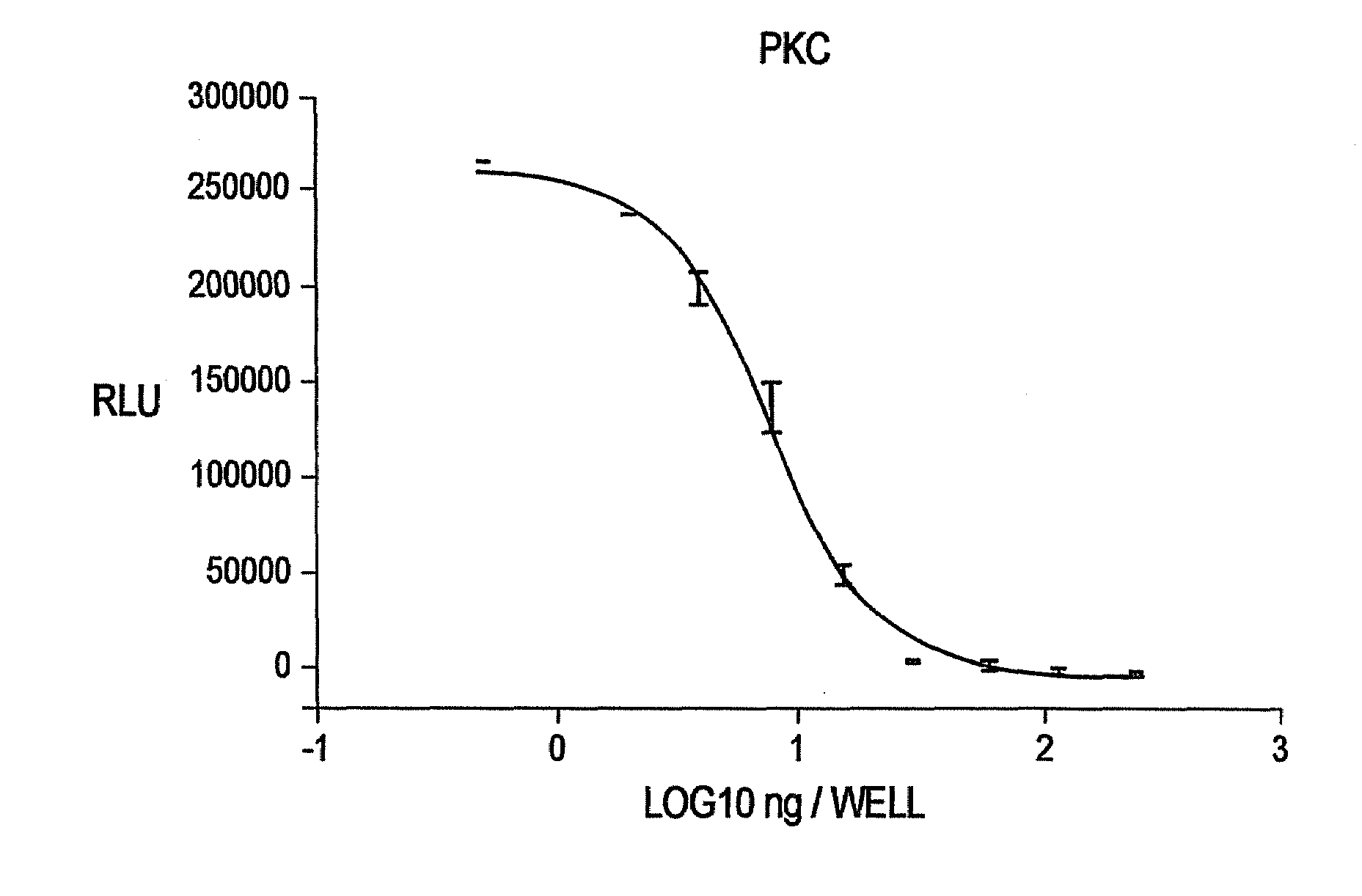
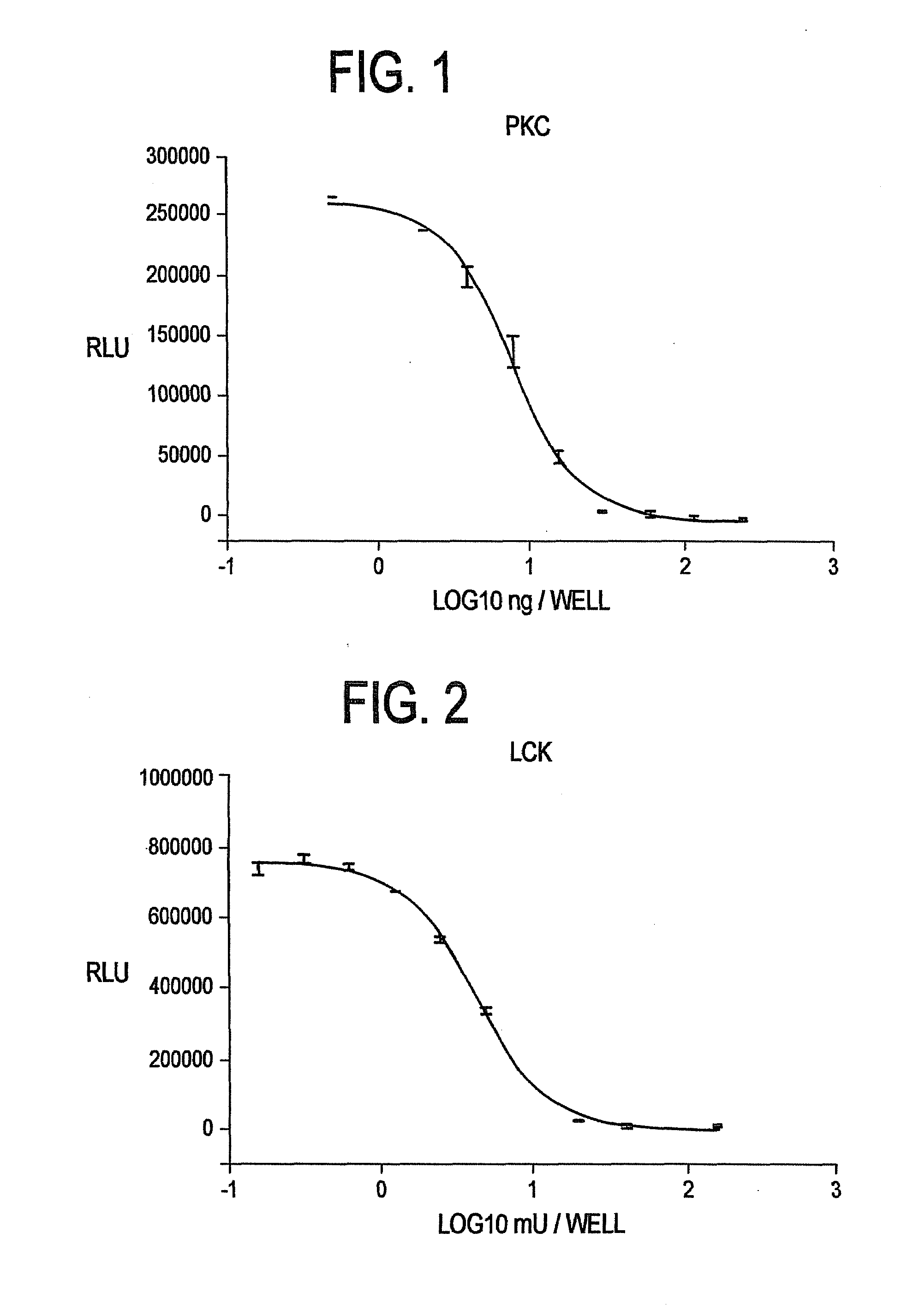
[0140]Commercially available Protein Kinase C (“PKC”) was titrated in a 96 well plate (n=2). Kinase reactions were performed in 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.1 mg / ml bovine serum albumin (“BSA”), 250 μM EGTA, 400 μM CaCl2, 0.32 mg / ml phosphatidylserine, 0.032 mg / ml diacylglycerol, 10 μM biotinylated peptide (AAKIQASFRGHMARKK), and 1 μM ATP with the indicated amount of PKC (Promega, Catalog #V5621). Final kinase reaction volume was 50 μl. Following a 90 minute kinase reaction, 50 μl of a luminescent buffer reagent (pH 6.0+0.15) containing 20 mM citrate; 55 mM MES; 10.5 mM magnesium sulfate; 0.6 mM CDTA; 225 mM potassium buffer (pH 6.0); 1 mM NaF; 0.0125 uM sodium pyrophosphate; 0.5% DTAB; 1.0% Thesit; 0.1% Mazu DF 204; 2.5 mM luciferin; and 0.2% 2-(N-morpholino) ethanesulfonic acid containing 10 μg / ml [TRUE?] of a thermostable firefly luciferase reporter 146-1H2 (see SEQ ID NO.: 4 and SEQ ID NO.: 8) was add...
example 2
Determining the Activity of Tyrosine Kinases
[0141]Commercially available Lck was titrated in a 96 well plate (n=2). Lck is a gene family encoding nonreceptor tyrosine kinases of the Src family. Kinase reactions were performed in 8 mM imidazole hydrochloride (pH 7.3), 8 mM β-glycerophosphate, 200 μM EGTA, 20 mM MgCl2, 1 mM MnCl2, 0.1 mg / ml BSA, 250 μM biotinylated peptide substrate (AEEEIYGELEA), and 3 μM ATP with the indicated amount of Lck (Upstate, Catalog #14-442). Final kinase reaction volume was 50 μl. Following a 60 minute kinase reaction, 50 μl of a luminescent buffer reagent as described in Example 1 was added to each well and allowed to incubate for 10 minutes prior to reading luminescence. FIG. 2 clearly shows that a consistent titration curve is obtained using the method of the present invention.
example 3
Determining the Activity of Cyclic-AMP Dependent Serine / Threonine Protein Kinase
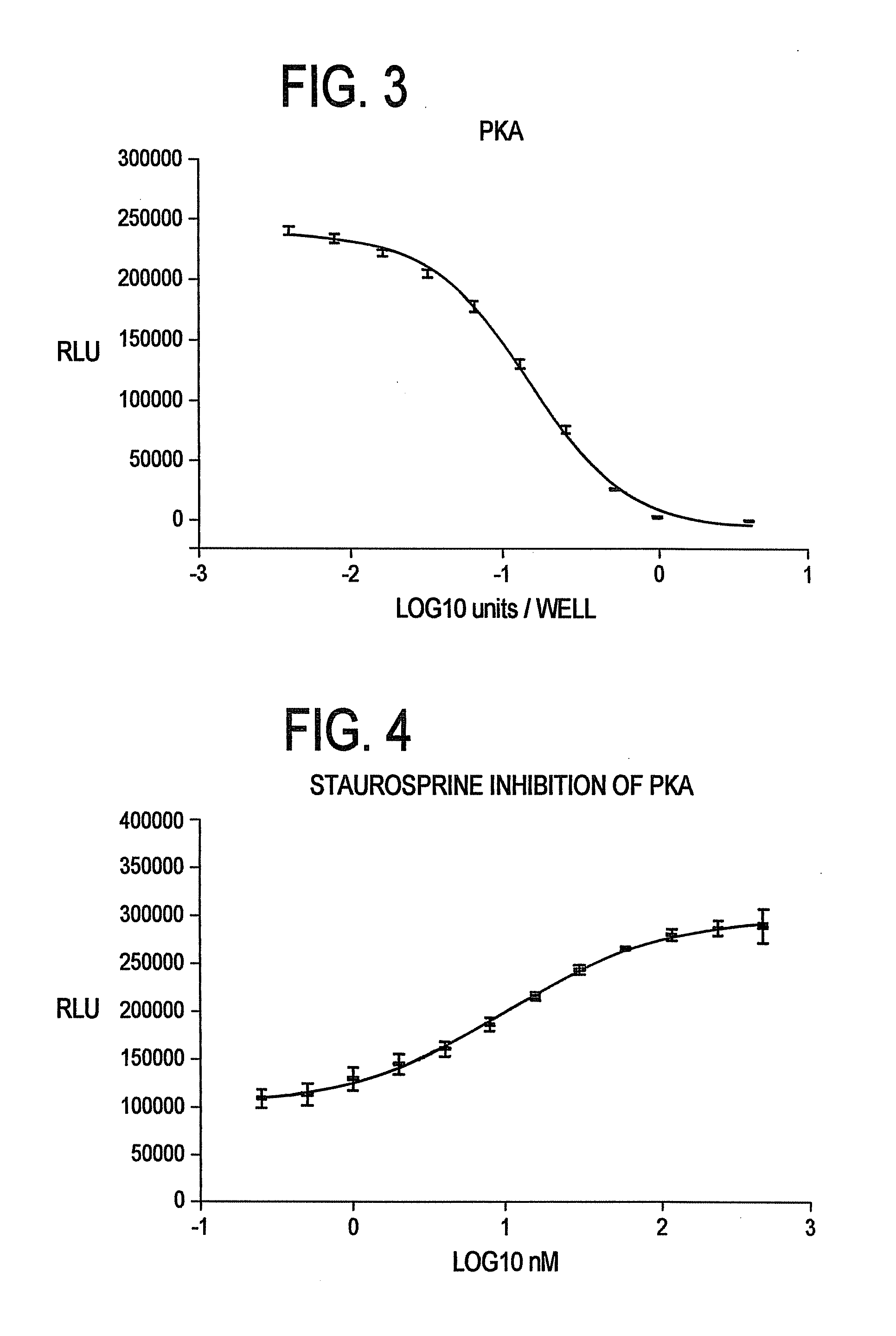
[0142]Commercially available Protein Kinase A (“PKA”) was titrated in a 96 well plate (n=8). Kinase reactions were performed in 40 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 0.1 mg / ml BSA, 5 μM kemptide (LRRASLG), and 1 μM ATP with the indicated amount of PKA (Promega, Catalog #V5161). Final kinase reaction volume was 50 μl. Following a 20 minute kinase reaction, 50 μl of a luminescent buffer reagent as described in Example 1 was added to each well and allowed to incubate for 10 minutes prior to reading luminescence. FIG. 3 clearly shows that using the method of the present invention, a reliable titration curve is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com