Monitoring and manipulating cellular transmembrane potentials using nanostructures
a technology of cellular transmembrane potential and nanostructure, which is applied in the direction of fluid pressure measurement, liquid/fluent solid measurement, peptide measurement, etc., can solve the problems of complex role of cell electrical properties in generating a defined disease phenotype, serious complications to health, and change of cell electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cells on Coverslips
[0131]Experiments were performed on A431 (a human cell line from an epidermoid carcinoma) cells or CHO (Chinese hamster ovary) cells stably expressing M1 muscarinic Gq-protein coupled receptor using nanoparticles commercially available from Quantum Dot Corp. (a wholly owned subsidiary of Invitrogen Corp.; Carlsbad, Calif.) and Evident Technologies (Troy, N.Y.). The intracellular (pipette) solution (pH 7.3) was composed of 140 mM CsCl, 10 mM EGTA, 10 mM HEPES. The extracellular solution (pH 7.4) was composed of 140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM EGTA, 10 mM glucose, 10 mM HEPES.
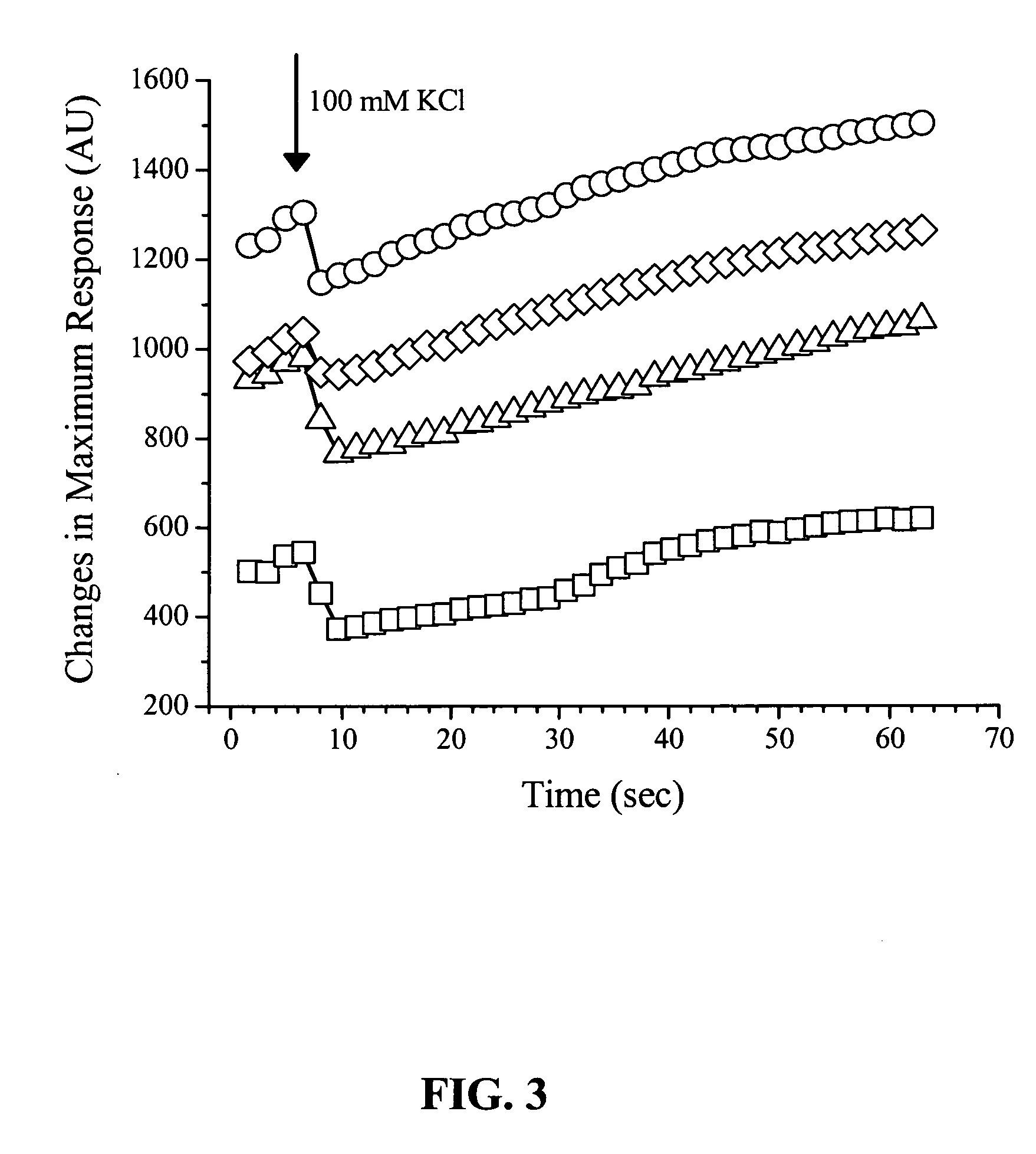
[0132]In experiments with extracellular delivery of nanoparticles, several types of commercially available nanocrystals were used. In one series of experiments, streptavidin-functionalized QD605 (Quantum Dot Corp.) in the buffer solution B from the QDot® kit were added to extracellular solution in concentrations from 25 to 500 μg / ml. In another experimental...
example 2
Use of Patch Pipette
[0136]Glass micropipettes for patch-clamp experiments were pulled from borosilicate glass capillaries (1.2 mm no-capillary glass, Sutter Instruments; Novato, Calif.) using a Sutter 2000™ pipette puller (model Sutter 2000; Sutter Instruments; Novato, Calif.) using the prerecorded 4-step patch pipette pulling protocol. The open diameter of the pipette tip was 1.5-2.2 μm with a resistance of 2-3 MΩ. The micropipettes were filled with intracellular solution.
[0137]Experiments were performed at room temperature in whole-cell patch-clamp configuration using a Axopatch200B patch-clamp amplifier (Molecular Devices; Sunnyvale, Calif.). After successful giga-seal formation, brief pulses of suction were used to rupture the cellular membrane to achieve whole-cell patch-clamp configuration.
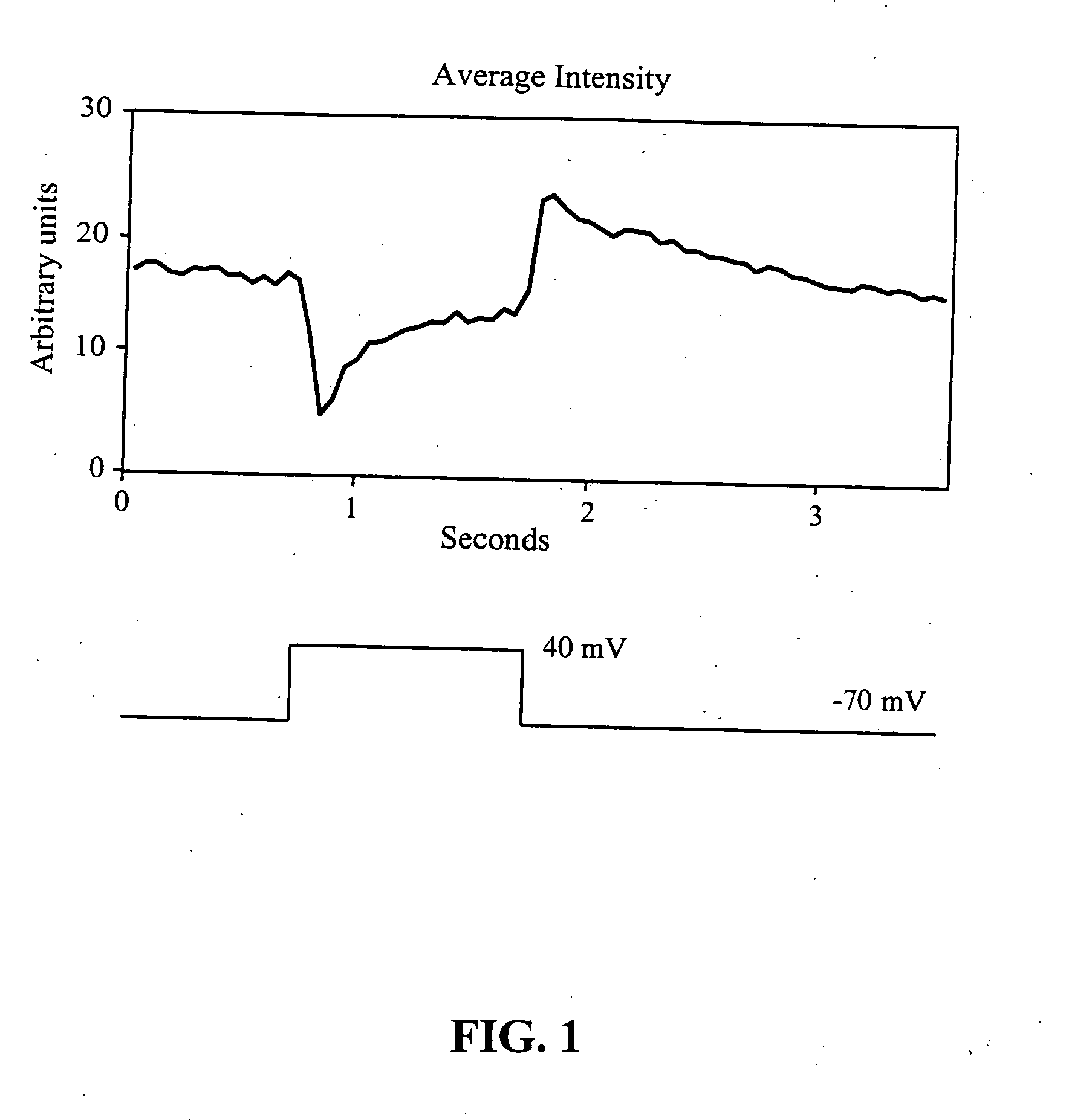
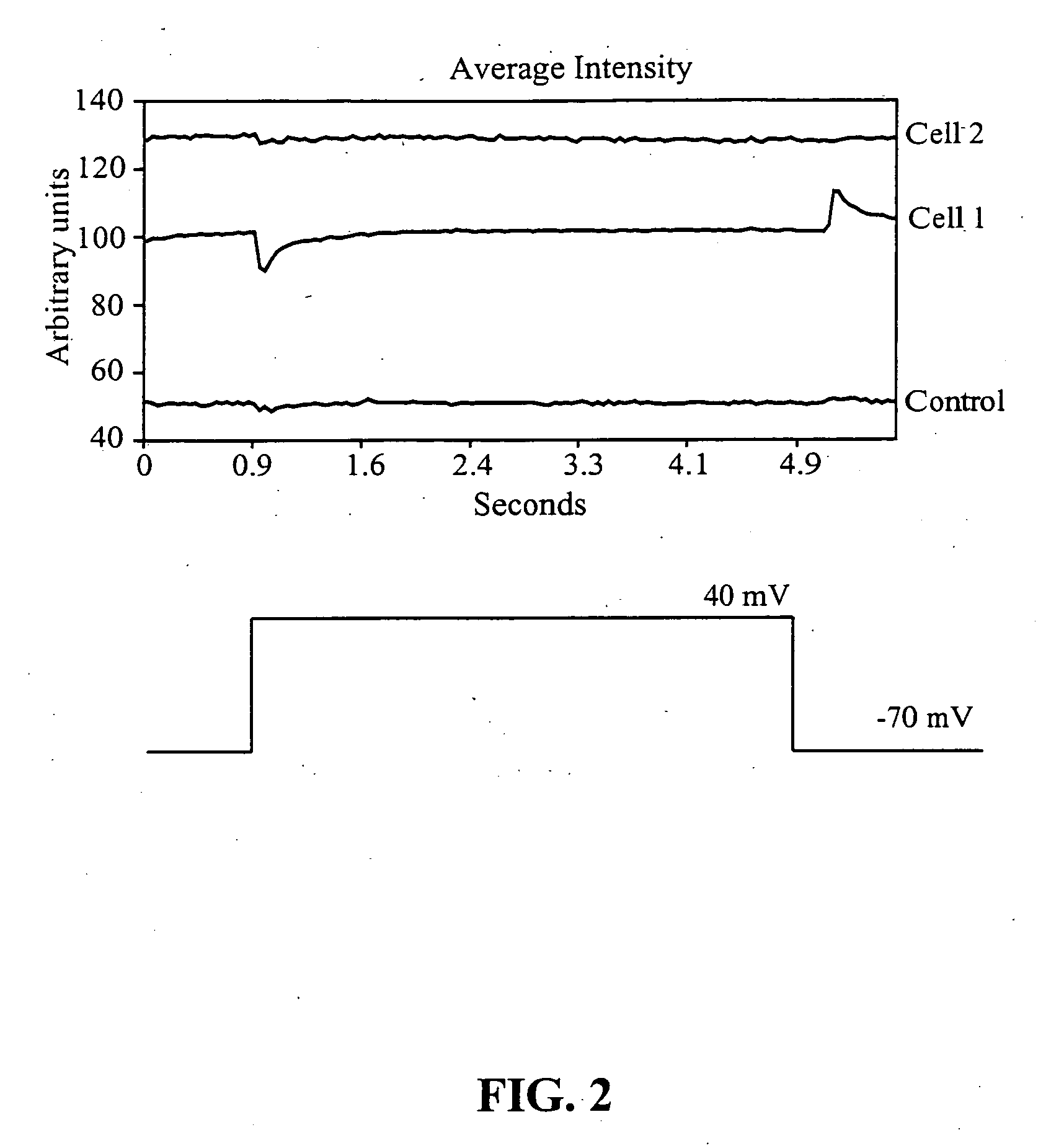
[0138]The following test protocol was used for cell stimulation. The membrane potential was set at −70 mV. A depolarizing pulse necessary to take the cell to +40 mV was applied to the interi...
example 3
External loading of streptavidin-coated nanoparticles
[0139]The emission intensity of externally applied streptavidin-functionalized nanoparticles occurring in response to voltage stimulation of the cell (QD605-streptavidin, Quantum Dot Corp.) was visualized using a cooled CCD Optronics Tec 470 camera (Optronic Engineering, Goleta, Calif.) linked to a computer. Voltage changes elicited across the cellular membrane via patch pipette attached to a cell did not result in changes in the emission intensity of these particular nanoparticles. Nine cells were tested in this series, and none exhibited changes in emission intensity to the voltage stimulation protocol described in the previous example. The streptavidin coating of the nanoparticles used in this example may have prevented the nanocrystals from being strategically placed inside the cellar membrane, the site of the highest membrane gradient. The streptavidin coating of the nanoparticles used in this example may have prevented the n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential / voltage | aaaaa | aaaaa |
| Electric potential / voltage | aaaaa | aaaaa |
| Electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com