Composition for treating atherosclerosis and a preparation method thereof
a technology of atherosclerosis and composition, which is applied in the field of composition, can solve the problems of renal hypertension and even uremia, side effects of stomach upset, diarrhea or constipation, etc., and achieve the effect of increasing the expression of ppar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
Experiment 1
Preparation of Chalcone Compounds
[0037]
[0038]Chalcone compound has ring A and ring B. For ease of illustration, the denotations of carbon atoms on ring A and ring B are shown as above.
[0039]The synthesis of the chalcone compounds in the present invention was carried out by a Claisen-Schmidt condensation. Taking chalcone compound 1 as the example, a mixture of 2-hydroxyacetophenone (273.6 mg, 2.01 mmol), 4-methoxybenzaldehyde (279.1 mg, 2.05 mmol), potassium hydroxide (KOH, 50% w / v, 2 ml) and ethanol (100% v / v, 20 ml) was stirred at room temperature for 24 hours. The reaction mixture was concentrated under reduced pressure and then partitioned with ethyl acetate (EtOAc) and H2O. The organic layer was then evaporated, and the residue was purified using column chromatography (silica gel: 70-230, Merck; n-hexane-EtOAc, 15:1, Rf=0.2) to give 2-hydroxy-4′-methoxychalcone (chalcone compound 1, 273.6 mg; yield, 53.6%). The purity of compound 1 was greater than 95%, which was det...
experiment 2
Activity of Chalcone Compound 1 and Other Flavonoid Compounds on Inhibiting HASMCs Proliferation
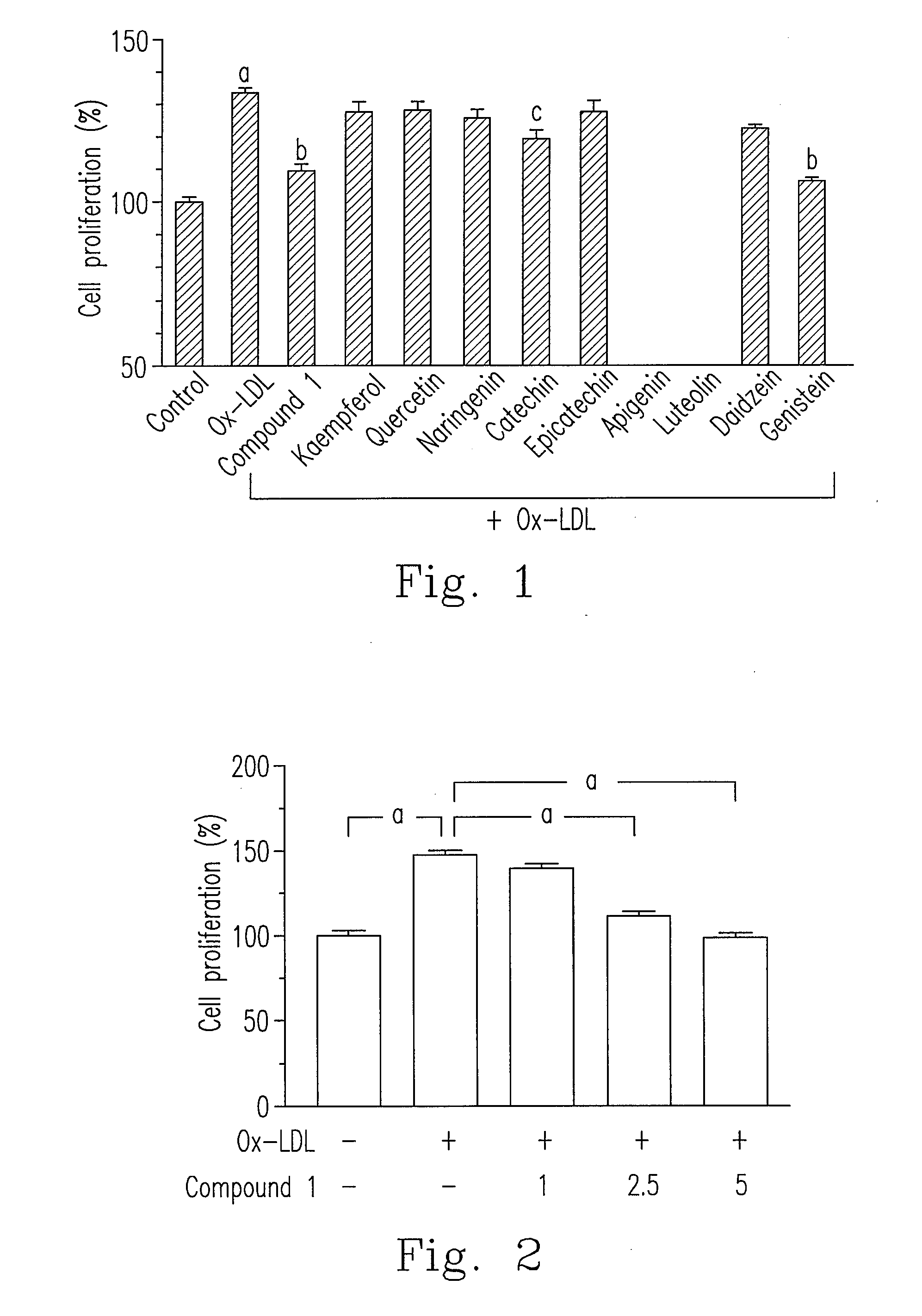
[0041]The skeleton of flavonoid compound is constructed by C6-C3-C6, and chalcone also belongs to this genus of structure. Generally speaking, the representative active flavonoid can be divided as six groups, including flavonol, flavanone, flavan-3-ol, flavone, isoflavone and anthocyanin. Followings are the basic skeleton in each genus of flavonoid compounds and the examples used in the present invention.
[0042]For analyzing whether each genus of flavonoid compounds could inhibit the activity of HASMCs proliferation, the proliferation and cell viability of HASMCs were determined using WST-1 reagent (Roche Applied Science, Penzberg, Germany). WST-1 is a tetrazolium salt that will be cleaved to formazan by mitochondrial dehydrogenases in viable cells. First, HASMCs were seeded into 96-well plates. After the density reaching 70% confluence, the cells were “synchronized” for 24 hours by replac...
experiment 3
Anti-Proliferation Effect of Compound 1 on HASMCs Stimulated with Ox-LDL
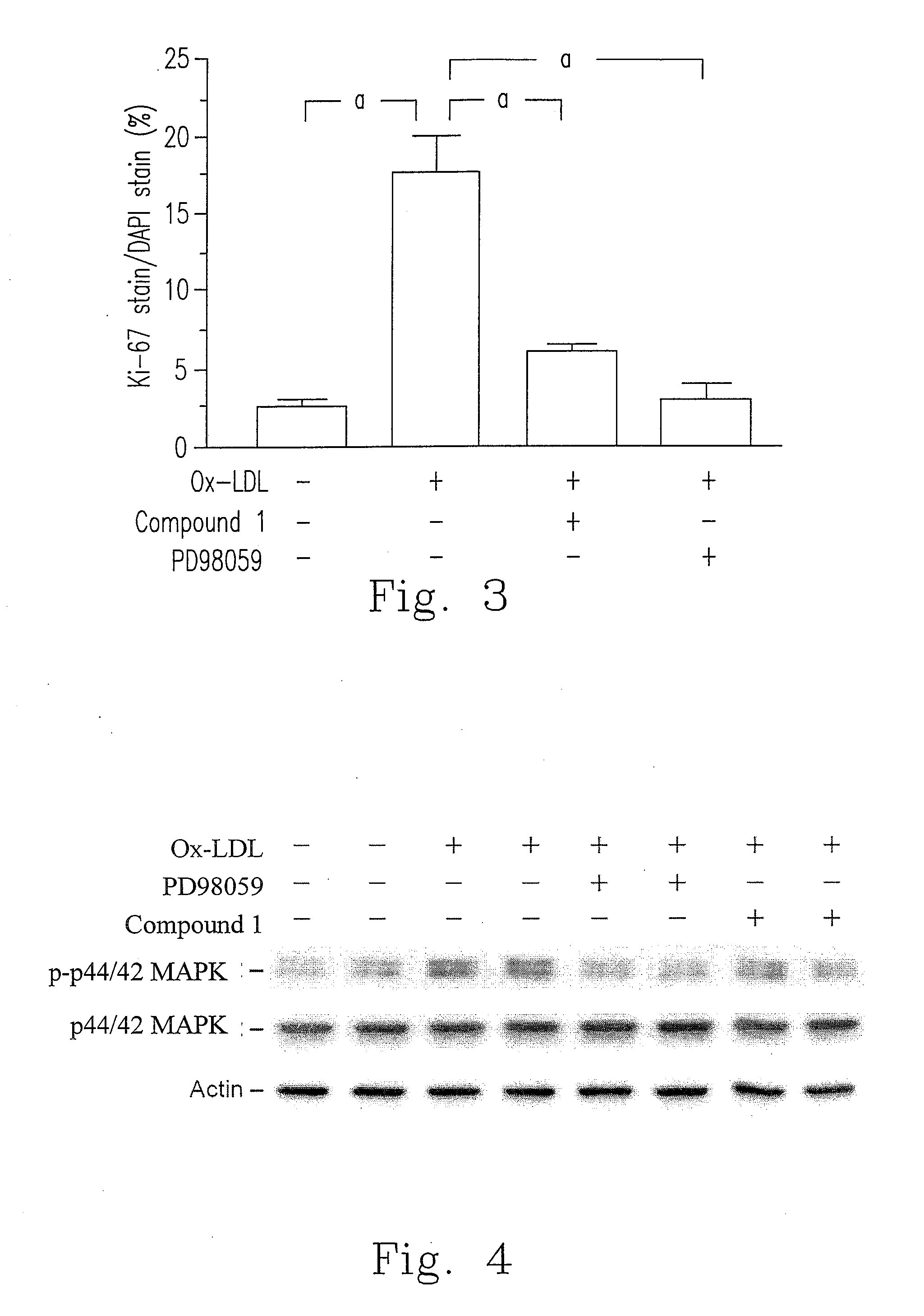
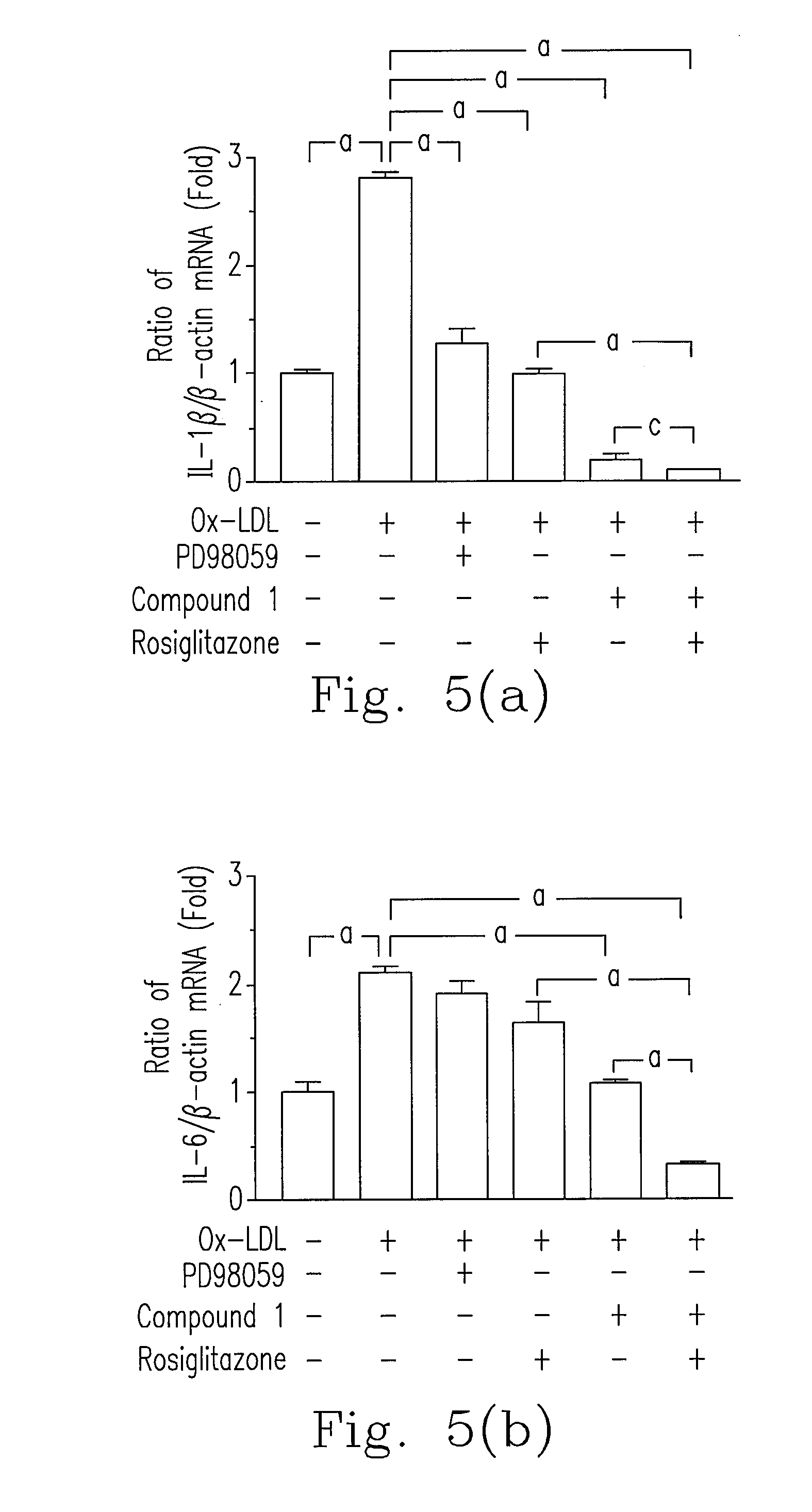
[0045]Please refer to FIG. 2, Ox-LDL (30 μg / ml) increased cell proliferation about 50% in HASMCs. As compared to Ox-LDL treatment group, cell proliferation of compound 1 treatment (1, 2.5 and 5 μg / ml) significantly decreased in a dose dependent manner.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com