Microorganisms and methods for production of specific length fatty alcohols and related compounds
a technology of fatty alcohol and microorganisms, applied in the direction of acyltransferases, dna/rna fragmentation, enzymes, etc., can solve the problems of not producing high yields of fatty alcohol, not commercially viable to produce fatty alcohol directly, and not producing fatty alcohol. achieve the effect of increasing the ratio of nad(p)h to nad(p)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
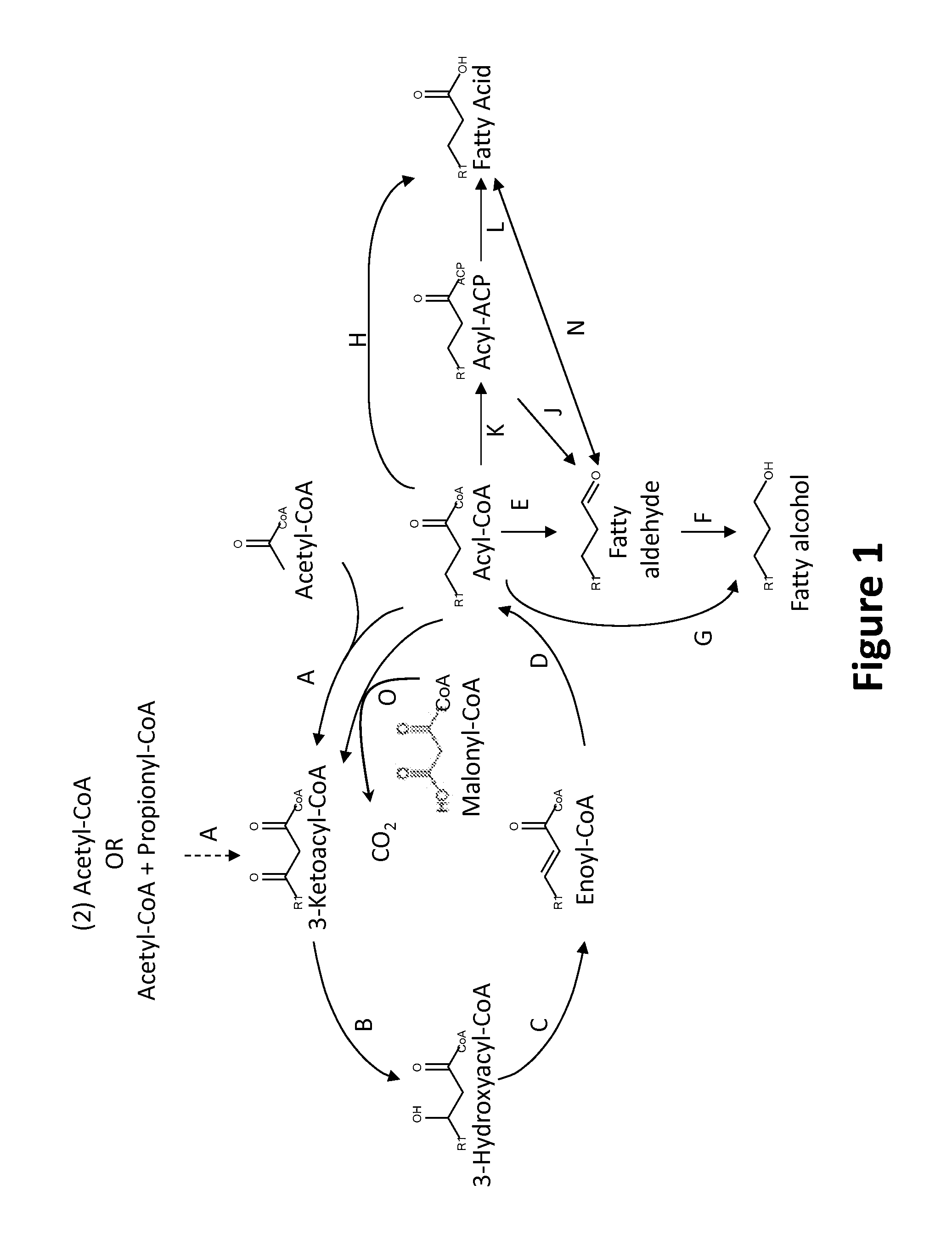
Production of Fatty Alcohols and Fatty Aldehydes by MI-FAE Cycle, MD-FAE Cycle and Acyl-CoA Termination Pathways
[0292]Encoding nucleic acids and species that can be used as sources for conferring fatty alcohol and fatty aldehyde production capability onto a host microbial organism are exemplified further below.
[0293]In one exemplary embodiment, the genes fadA and fadB encode a multienzyme complex that exhibits three constituent activities of the malonyl-CoA independent FAS pathway, namely, ketoacyl-CoA thiolase, 3-hydroxyacyl-CoA dehydrogenase, and enoyl-CoA hydratase activities (Nakahigashi, K. and H. Inokuchi, Nucleic Acids Research 18:4937 (1990); Yang et al., Journal of Bacteriology 173:7405-7406 (1991); Yang et al, Journal of Biological Chemistry 265:10424-10429 (1990); Yang et al., Biochemistry 30:6788-6795 (1990)). The fadI and fadJ genes encode similar activities which can substitute for the above malonyl-CoA independent FAS conferring genes fadA and fad...
example ii
Pathways for Producing Cytosolic Acetyl-CoA from Cytosolic Pyruvate
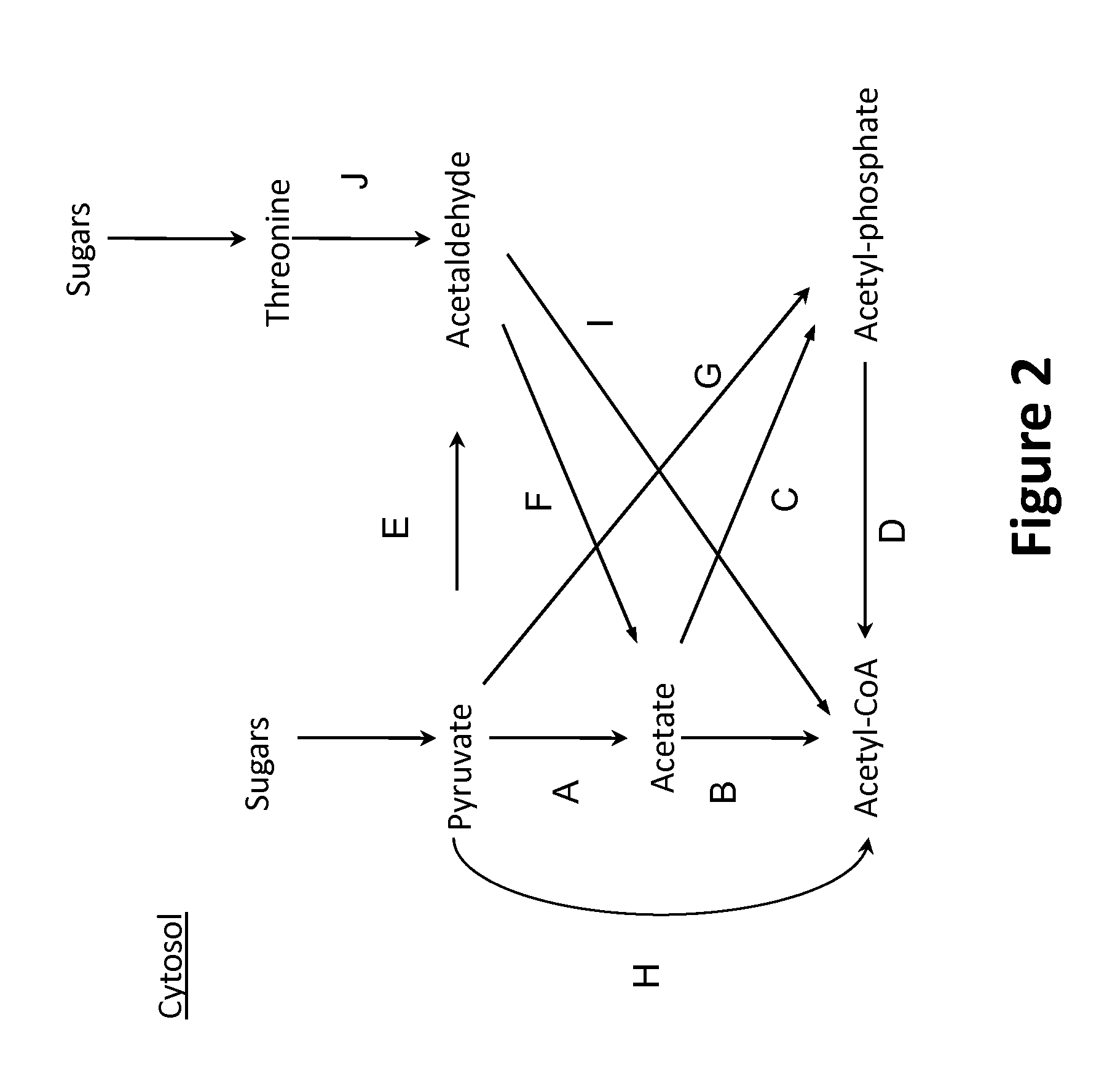
[0331]The following example describes exemplary pathways for the conversion of cytosolic pyruvate and threonine to cytosolic acetyl-CoA, as shown in FIG. 2.
[0332]Pathways for the conversion of cytosolic pyruvate and threonine to cytosolic acetyl-CoA could enable deployment of a cytosolic fatty alcohol, fatty aldehyde or fatty acid production pathway that originates from acetyl-CoA. Several pathways for converting cytosolic pyruvate to cytosolic acetyl-CoA are shown in FIG. 2. Direct conversion of pyruvate to acetyl-CoA can be catalyzed by pyruvate dehydrogenase, pyruvate formate lyase, pyruvate:NAD(P) oxidoreductase or pyruvate:ferredoxin oxidoreductase. If a pyruvate formate lyase is utilized, the formate byproduct can be further converted to CO2 by formate dehydrogenase or formate hydrogen lyase.
[0333]Indirect conversion of pyruvate to acetyl-CoA can proceed through several alternate routes. Pyruvate can be convert...
example iii
Pathways for Producing Acetyl-CoA from PEP and Pyruvate
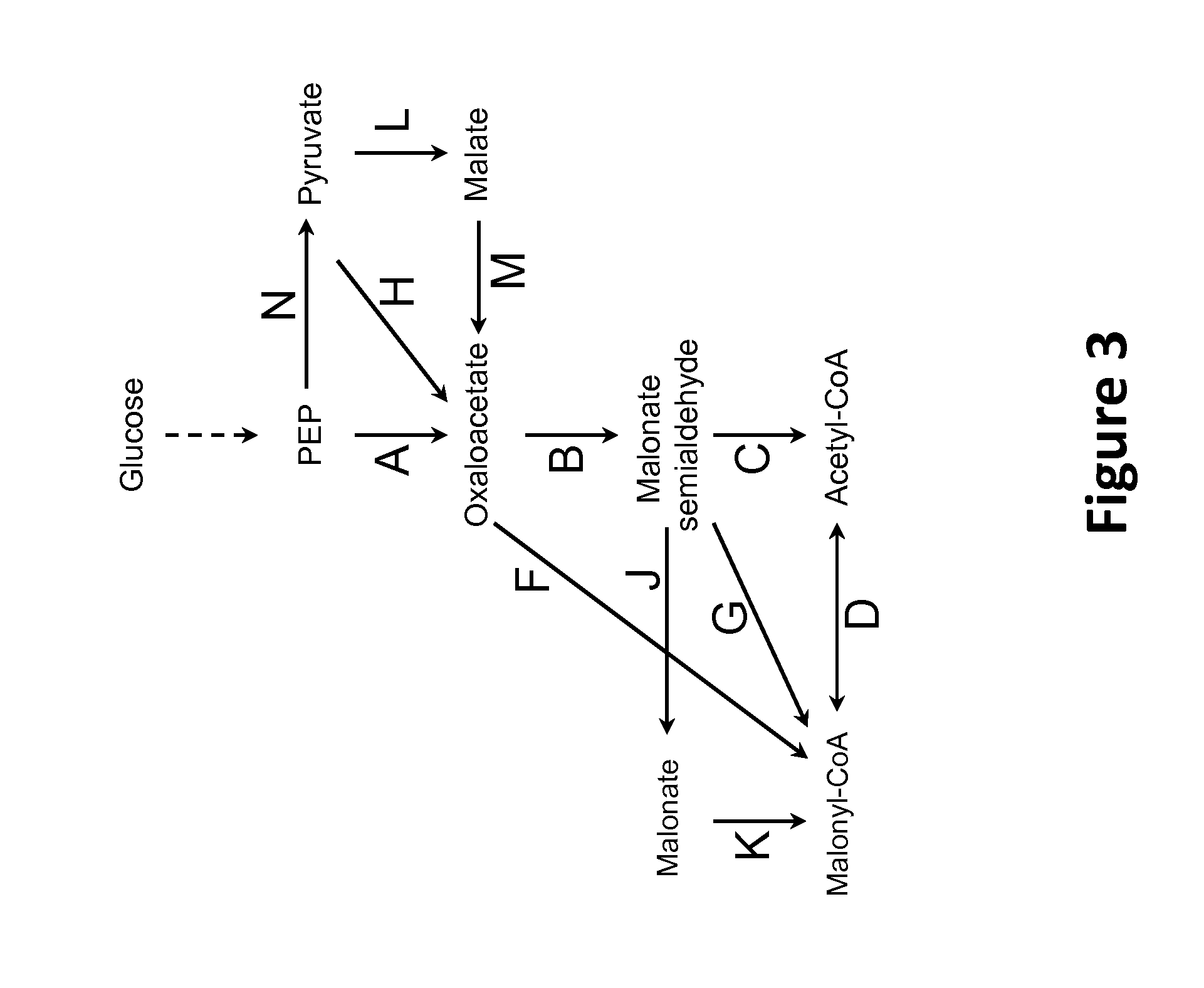
[0352]Pathways for the conversion of cytosolic phosphoenolpyruvate (PEP) and pyruvate to cytosolic acetyl-CoA can also enable deployment of a cytosolic fatty alcohol, fatty aldehyde or fatty acid production pathway from acetyl-CoA. FIG. 3 shows numerous pathways for converting PEP and pyruvate to acetyl-CoA.
[0353]The conversion of PEP to oxaloacetate is catalyzed in one, two or three enzymatic steps. Oxaloacetate is further converted to acetyl-CoA via malonate semialdehyde or malonyl-CoA intermediates. In one pathway, PEP carboxylase or PEP carboxykinase converts PEP to oxaloacetate (step A); oxaloacetate decarboxylase converts the oxaloacetate to malonate (step B); and malonate semialdehyde dehydrogenase (acetylating) converts the malonate semialdehyde to acetyl-CoA (step C). In another pathway pyruvate kinase or PEP phosphatase converts PEP to pyruvate (step N); pyruvate carboxylase converts the pyruvate to (step H); oxaloacet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| excitation wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
| enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com