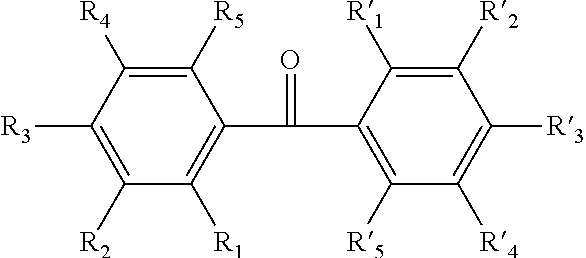
Uses of hydroxybenzophenone in preparation of antiviral and antitumor drugs
a technology of hydroxybenzophenone and antiviral and antitumor drugs, which is applied in the direction of drug compositions, viruses/bacteriophages, and dsdna viruses, can solve the problems of lack of effective drugs for treating genital warts, significant differences in structure of hydroxybenzophenone, and increase the risk of suffering from alzheimer's disease in individuals, so as to prevent the recurrence of viral infection and high antiviral and antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Anti-HPV Microemulsion
[0067]20 ml 1,2-propylene glycol, 15 ml Tween 80 and 5 ml ketone are mixed and added with sterilized distilled water to a total volume of 100 ml, so as to obtain an external preparation solution. 10 g 2,4,2′,4′-tetrahydroxybenzophenone is added to 50 ml of the external preparation solution, the pH thereof is adjusted to 5.5, and then the solution is added with the external preparation solution to 100 ml, so as to obtain 10% anti-HPV microemulsion of the present invention.
example 2 preparation
of Anti-HPV 2,3,4,2′,4′,5-hexahydroxybenzophenone Injection
[0068]10 g 2,3,4,2′,4′,5-hexahydroxybenzophenone, 8.5 g sodium chloride, 10 ml 1,2-propanediol and 80 ml Tween 80 are mixed and added with the sterilized distilled water to be dissolved, then added with the sterilized distilled water to 100 ml, the pH is adjusted to 7.4, and then the solution is filtered, potted and sterilized at 100° C. for 30 mins. Consequently, 2,3,4,2′,4′,5-hexahydroxybenzophenone injection is obtained.
example 3
Preparation of 2,3,4,2′,4′,5-hexahydroxybenzophenone Compound Effervescent Tablets
[0069]0.25 g 2,3,4,2′,4′,5-hexahydroxybenzophenone, 0.01 g polyinoside and 0.45 g tartaric acid are screened separately by 80 mesh sieve, and prepared with anhydrous ethanol into damp mass, and then screened by 12 mesh sieve to obtain wet granules, then the wet granules are dried at 50° C. for use. Besides, 0.65 g sodium bicarbonate and 0.02 g dextrin in addition with the sterilized distilled water are prepared into damp mass, and are screened by 12 mesh sieve to obtain wet granules, then the wet granules are dried at 50° C. These granules are mixed with the above dry granules, and the mixed granules are granulated, added with an appropriated amount of sterilized distilled water, baked for a while, added with 0.01 g PEG6000, uniformly mixed, and finally compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com