Directed evolution of cyp52a12 gene and its use in dicarboxylic acid production
a technology of cyp52a12 and dicarboxylic acid, which is applied in the field of preparing a long-chain dicarboxylic acid producing strain, can solve the problems of huge obstacles to serious affecting the development of long-chain dicarboxylic acid in the industry, and many challenges in chemical synthesis. achieve the effect of significantly shortening the fermentation cycl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
edia and Methods for Culture and Fermentation as Well as for Detecting a Dicarboxylic Acid
[0086]1. YPD medium formula (w / v) was: 2% peptone, 2% glucose and 1% yeast extract (OXOID, LP0021). 1.5-2% agar powder was added to form a solid media.
[0087]During culturing, a single colony was picked in a 2 ml centrifuge tube containing 1 ml YPD liquid medium, incubated at 30° C. in a 250 RPM shaker for 1 day.
[0088]2. Seed medium formula (w / v): sucrose 10 to 20 g / L, yeast extract 3 to 8 g / L, industrial fermentation corn syrup (for short, corn syrup, with total nitrogen content 2.5 wt %) 2 to 4 g / L, KH2PO4 4 to 12 g / L, urea 0.5 to 4 g / L (separately sterilized at 115° C. for 20 min), and the substrate for fermentation was n-dodecane 20 mL / L.
[0089]During culturing, the inoculum obtained in step 1 was inoculated into a 500 mL shake flask containing 30 mL seed medium, wherein the amount of inoculum was 3-5%, and incubated at 30° C. in a 250 RPM shaker until OD620 reached 0.8 (after 30-fold dilutio...
example 2
on of CYP52A12 Mutation Template
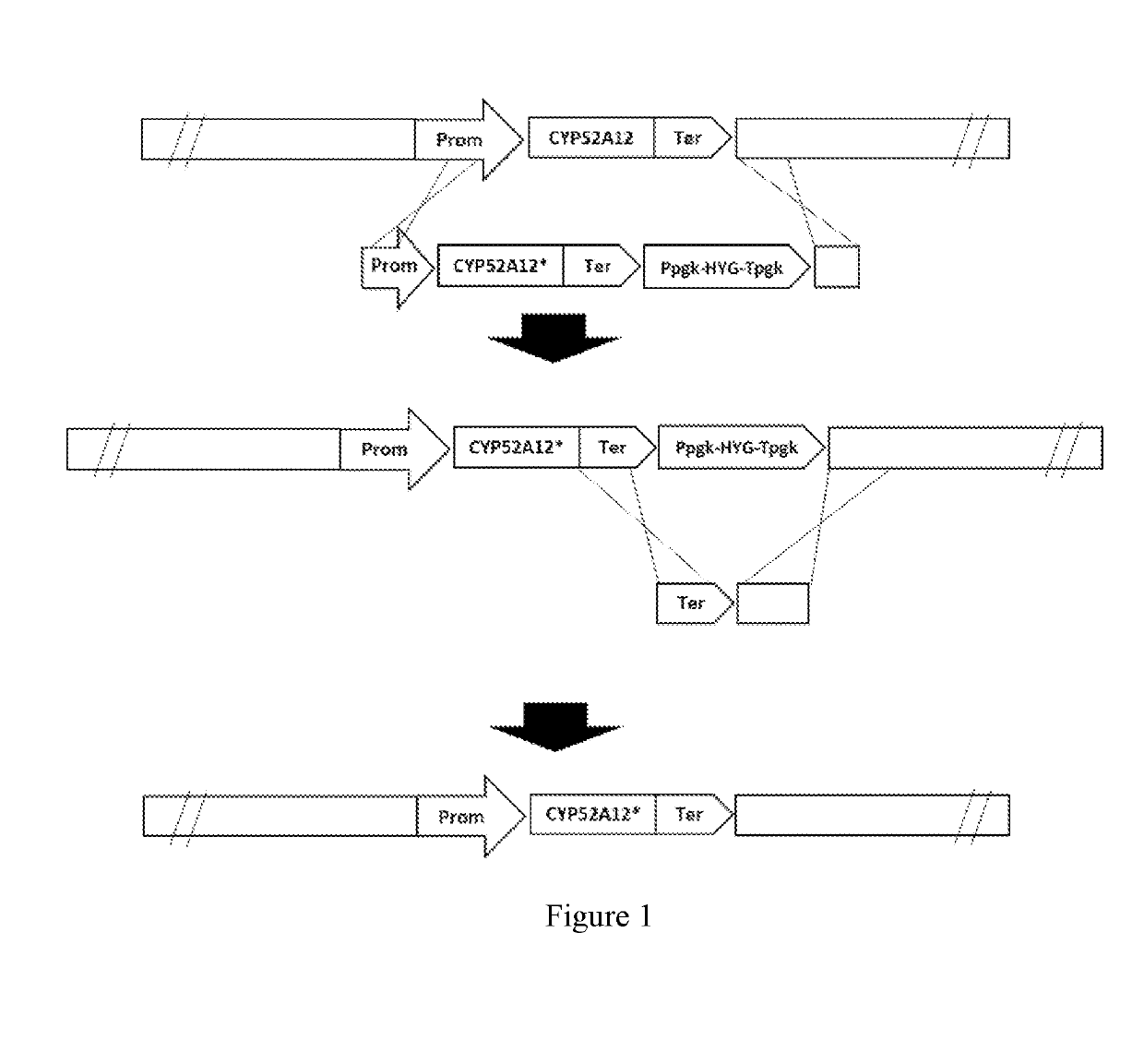
[0094]1. Preparation of the CYP52A12 mutation template.
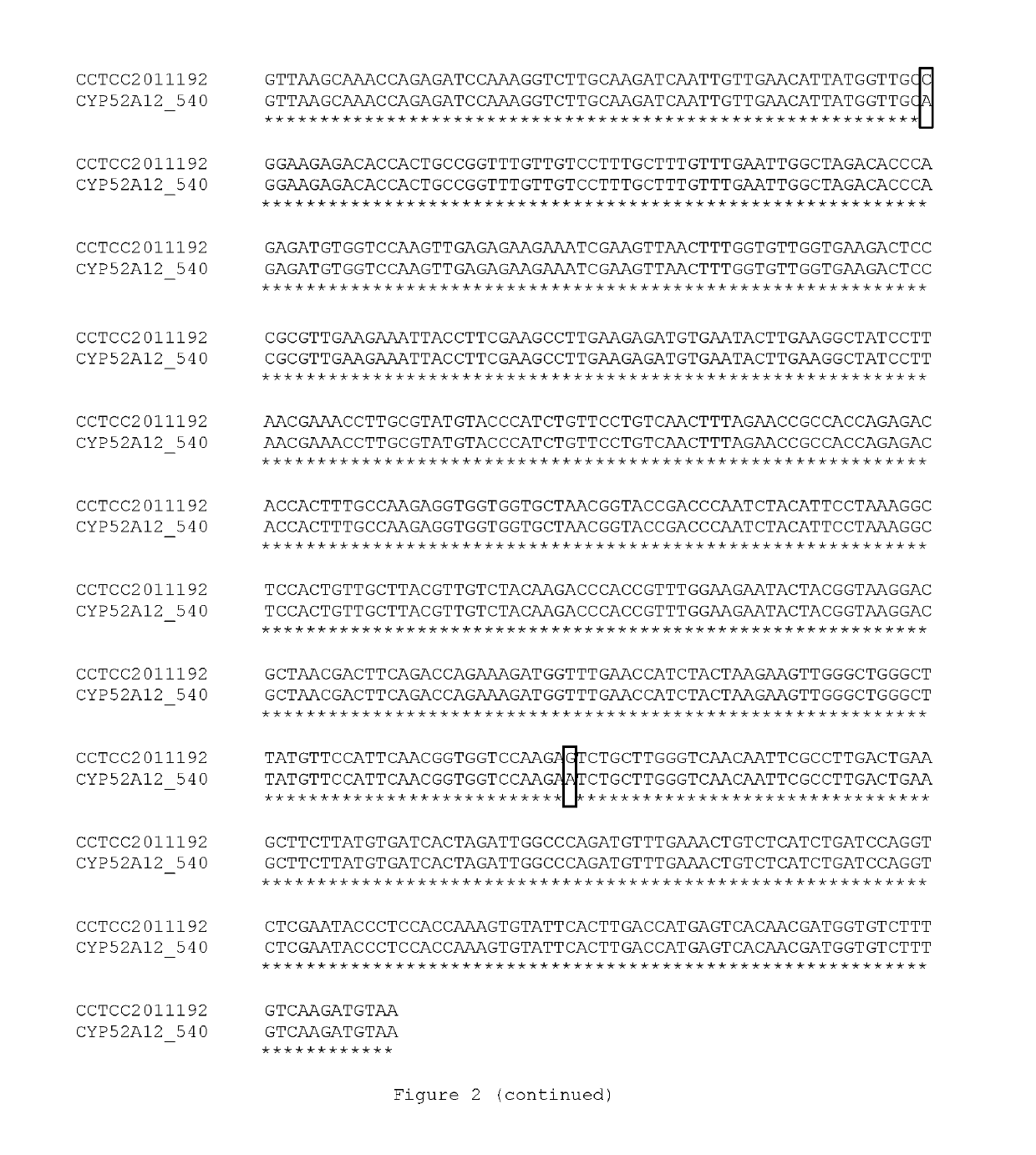
[0095]The genomic DNA of Candida CCTCC M 2011192 was extracted by using Ezup Yeast Genomic DNA Extraction Kit (Sangon, Cat No. 518257). A method with liquid nitrogen grinding was used in favor of increasing the cell wall disruption efficiency. Genomic DNA obtained by this method was used as template for error-prone PCR.
[0096]2. Error-prone PCR
[0097]The concentration of Mg2+ was adjusted (2-8 mM) and the CYP52A12 gene was amplified by error-prone PCR using Taq DNA Polymerase (Takara, Cat No. R00113).
[0098](PCR condition was: Step 1: 98° C. for 30 s, step 2: 98° C. for 10 s, 55° C. for 30 s, 72° C. for 2 m 20 s, 35 cycles in total, Step 3: 72° C. for 5 m).
[0099]The primers were as follows:
CYP52A12-F:(SEQ ID NO: 1)5′-CAAAACAGCACTCCGCTTGT-3′,CYP52A12-R:(SEQ ID NO: 2)5′-GGATGACGTGTGTGGCTTGA-3′,
[0100]The PCR product was subjected to electrophoresis on a 1% agarose gel and recovered and purified by using...
example 3
on of Homologous Recombination Template
[0101]All DNA fragments in this example were obtained by amplification using PrimeSTAR® HS High Fidelity DNA polymerase (Takara, R040A). The DNA fragments were subjected to electrophoresis on a 1% agarose gel, followed by recovery and purification by using the Axygen Gel Recovery Kit.
[0102](1) Amplification of the resistance selection marker (HYG, the hygromycin resistance gene). The amplification template was the vector pCIB2 (SEQ ID NO: 3) owned by our company. The primer sequences were as follows:
CYP52A12_HYG-F:(SEQ ID NO: 4)5′-TCAAGCCACACACGTCATCCGCATGCGAACCCGAAAATGG-3′,CYP52A12_HYG-R:(SEQ ID NO: 5)5′-GATGTGGTGATGGGTGGGCTGCTAGCAGCTGGATTTCACT-3′.
[0103]The PCR reaction condition was as follows:
[0104]Step 1: 98° C. for 30 s,
[0105]Step 2: 98° C. for 10 s, 55° C. for 30 s, 72° C. for 1 m 50 s, 5 cycles,
[0106]Step 3: 98° C. for 10 s, 72° C. for 2 m, 25 cycles,
[0107]Step 4: 72° C. for 5 m.
[0108]The resulting product, named HYG, was verified by seq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com