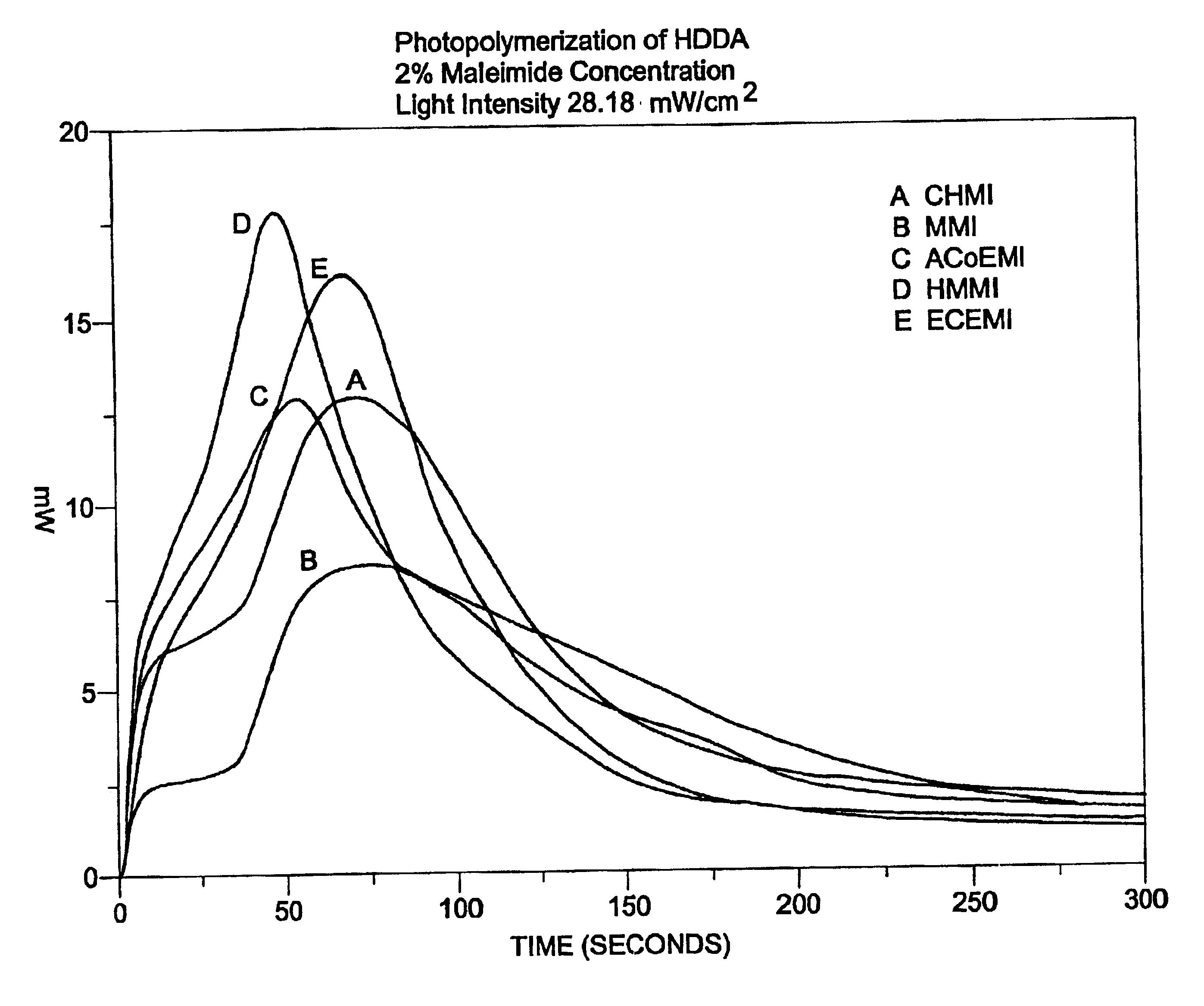
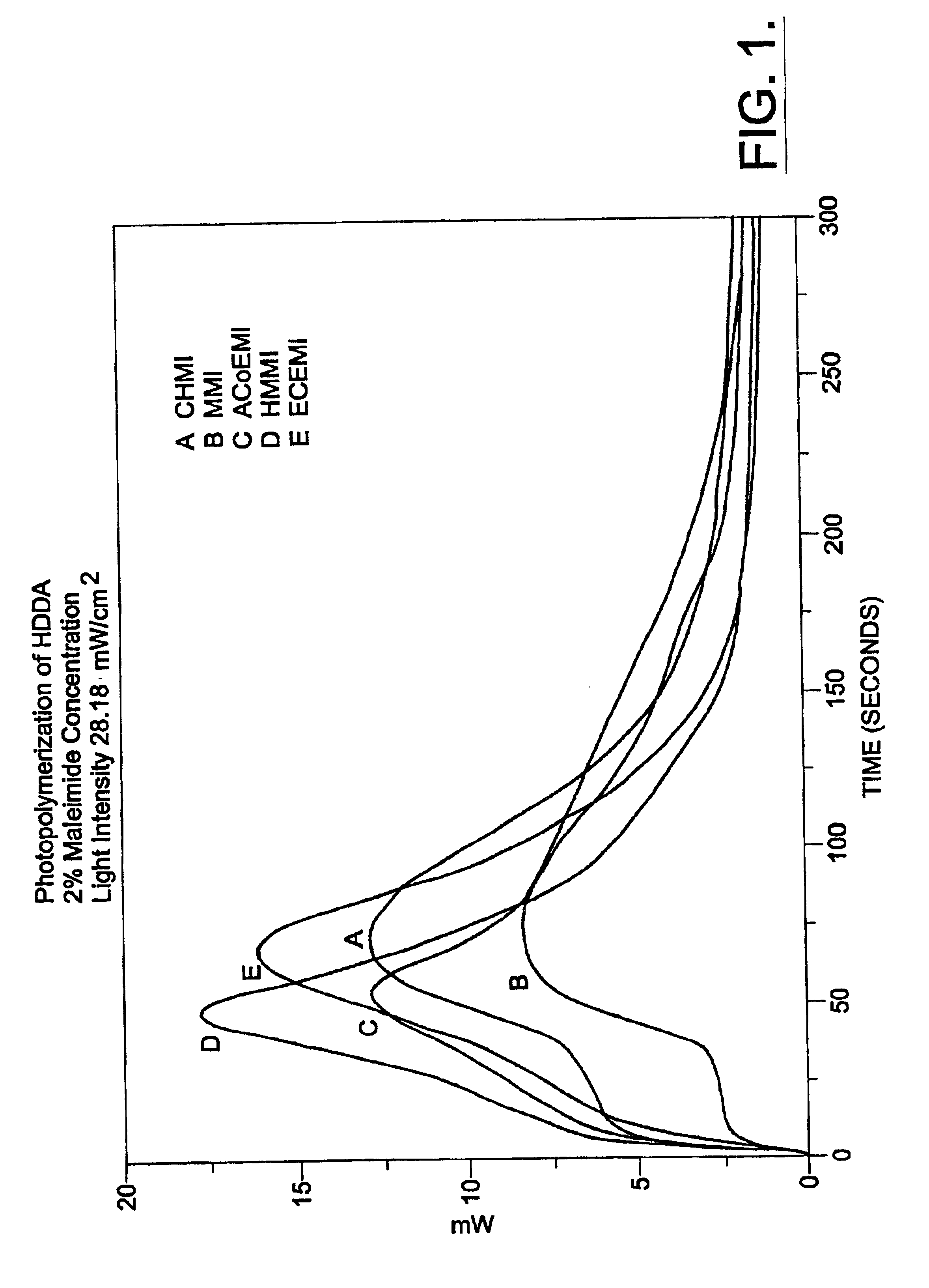
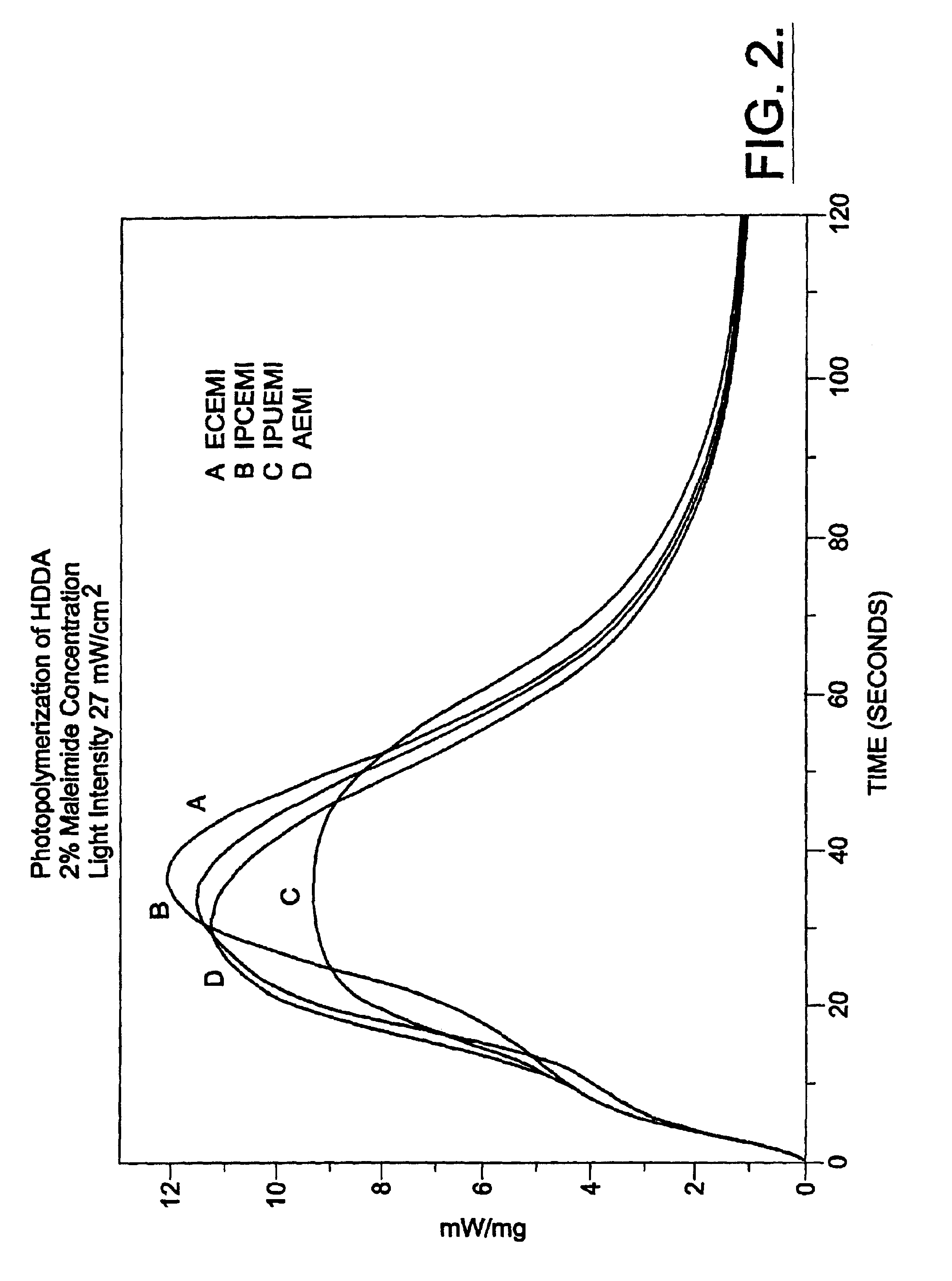
Polymerization processes using aliphatic maleimides
a technology of aliphatic maleimides and polymerization processes, which is applied in the field of maleimide compounds, can solve the problems of reducing the resistance to oxidative degradation, discoloration, and degradation of the physical properties of the article, and achieves the effects of increasing brittleness, minimal degradation over time, and thick coatings of polymerizable compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Hydroxy Methylmaleimide (HMMI)
Maleimide (10 g, 0.103 mol) was added to 10 mL of a 37% solution of formaldehyde and 0.31 mL of a 5% solution of NaOH was added. Within 10 minutes all of the maleimide had dissolved and an exothermic reaction proceeded. The solution was stirred for 2 hours where white crystals were observed. The solution was placed in a freezer overnight and the resulting crystals filtered and washed with ice cold ethanol and diethyl ether. The white crystals were purified twice by sublimation. See P. O. Tawney, R. H. Snyder, R. P. Conger, K. A. Leibbrand, C. H. Stiteler, and A. R. Williams J. Org. Chem. 26, 15 (1961). m.p. 104-106° C. (9.77 g, 74.6%). 1H-NMR (Acetone-d6, δ, ppm): 4.96 (2H, —CH2—, s), 5.33 (1H, —OH, s), 6.93 (2H, —CH═CH—, s). 13C-NMR (Acetone-d6, δ, ppm): 60.9 (1C, —CH2—), 135.6 (2C, —CH═CH—), 173.1 (1C, —C═O).
example 2
Synthesis of Hydroxy Ethylmaleimide (HEMI)
Ethanolamine (80.96 g, 1.32 mol) was added to 500 mL of ethanol and cooled to 0° C. using an ice bath. 3,6-Endoxo-1,2,3,6-tetrahydrophthalic anhydride (220.21 g, 1.32 mol) was added to the solution and allowed to stir overnight. The yellow tinted crystals were used without purification. The solution was refluxed for four hours with azeotropic removal of water. The solution was cooled to 0° C. and the resulting crystals filtered (151.74 g, 54.95%). Removal of furan was facilitated by refluxing the crystals in xylenes for 4 hours with quantitative yield of hydroxy ethylmaleimide after purification by sublimation to yield white crystals, —CH2O—), 134.2 (2C, —CH═CH—), 171.2 (2C, —NC═O m.p. 68° C. 1H-NMR (CDCl3, δ, ppm): 2.62 (1H, —OH, s), 3.82-3.77 (4H, —NCH2CH2O—, overlapping), 6.76 (2H, —CH═CH—, s). 13C-NMR (CDCl3, δ, ppm): 40.5 (1C, —NCH2—), 60.5 (1C,).
example 3
Triethylene Glycol Biscarbonate Bisethylmaleimide (TEGBCBEMI)
HEMI (25.65 g, 0.182 moles) and pyridine (14.38 g, 0.182 moles) were dissolved in THF (130 mL) and the solution was stirred at room temperature. Triethylene glycol bischloroformate (25.0 g, 0.091 moles) was added dropwise and stirred for 90 minutes. The pyridine salt was filtered off and the solution was combined with 200 mL of a 1N HCl solution. The product was extracted with methylene chloride and washed with a 1N HCl solution followed by water and then dried over magnesium sulfate. The red solution was diluted to a volume of 150 mL and purified by column chromatography (2.5 cm×21 cm) using silica gel as the packing and methylene chloride as the mobile phase yielding white crystals, m.p. 65° C. (26.75 g, 60.76%). 1H-NMR (CDCl3), δ, ppm): 3.64-3.68 (4H φ-OCH2—, t), 3.69-3.74 (4H, ε-OCH2—, t), 3.81-3.86 (4H, —NCH2—, t), 4.26-4.3 (8H, —CH2O(C═O)OCH2—, t), 6.75 (4H, —CH═CH—, s). 13C-NMR (CDCl3, δ, ppm): 36.6 (2C, —NCH2—), 64...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com