Cepharanthine oral disintegration tablet and its preparing method
A technology of orally disintegrating tablets and stephadin, which is applied in the field of stephadin orally disintegrating tablets, can solve the problems of low bioavailability, inconvenient taking, and affecting the effect of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
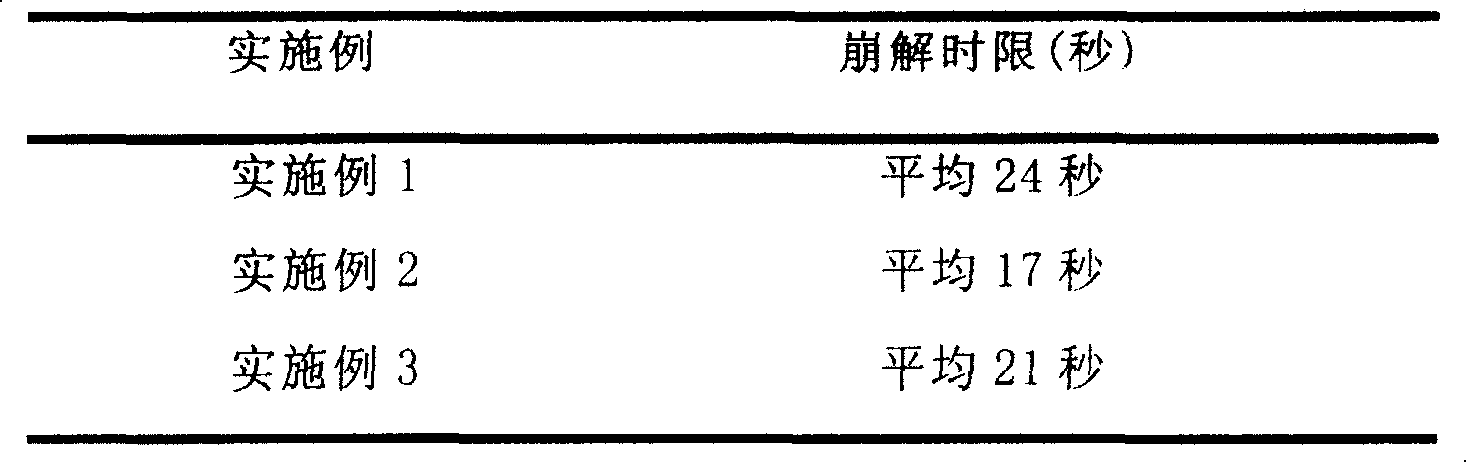
Embodiment 1
[0038] 1. Prescription
[0039] 1. Raw material - 20.0g of stephadin;
[0040] 2. Adhesive - polyvinylpyrrolidone K-30 3.0g;
[0041] 3. Filling agent - mannitol 80.0g;
[0042] Microcrystalline Cellulose 10.0g
[0043] Starch 10.0g
[0044] 4. Flavoring agent - aspartame 1.0g;
[0045] Ginseng essence 1.0g;
[0046] 5. Disintegrant—cross-linked polyvinylpyrrolidone 12.0g;
[0047] Low-substituted hydroxypropyl cellulose 16.0g;
[0048] 6. Glidant—micronized silica gel 1.0g;
[0049] 7. Lubricant—magnesium stearate 1.0g.
[0050]
[0051] A total of 1000 pieces were made
[0052] 2. Preparation method
[0053] 1) Mix the raw material of pachyphyllin with starch, make a soft material with a 70% aqueous solution containing 20% polyvinylpyrrolidone K-30, pass through a 30-mesh sieve to granulate, dry, and granulate with a 30-mesh sieve for later use;
[0054] 2) Mix mannitol, microcrystalline cell...
Embodiment 2
[0056] Example 2 The taste-masking method of drug coating on pellets
[0057] 1. Prescription
[0058] 1. Raw material - stephadin 200.0g;
[0059] 2. Blank pellet core (grain diameter: 200 microns) 160.0g;
[0060] 4. Adhesive - PVP k30 Ethanol solution with PVP k30 20.0g;
[0061] 5. Coating material—— E100 18.0g;
[0062] NE30D (dry) 4.0g;
[0063] 6. Filling agent - mannitol 600.0g;
[0064] Microcrystalline cellulose 100.0g;
[0065] 7. Flavoring agent - aspartame 5.0g;
[0066] Orange essence 5.0g;
[0067] 8. Disintegrant—cross-linked polyvinylpyrrolidone 100.0g;
[0068] Low-substituted hydroxypropyl cellulose 75.0g;
[0069] 9. Glidant - micronized silica gel 10 0g;
[0070] 10. Lubricant—sodium stearyl fumarate 10.0g.
[0071]
[0072] A total of 10,000 pieces were made
[0073] 2. Preparation method
[0074] 1. Make PVP-k30 into 10% ethanol solution, add stepherin to dissolve and set aside...
Embodiment 3
[0079] Example 3 Stirring granulation mechanism Pellet coating taste masking method
[0080] 1. Prescription
[0081] 1. Raw material - stephadin 200.0g;
[0082] 2. Accessory material - starch 200.0g
[0083] Dextrin 60.0g
[0085] Magnesium Stearate 2.0g
[0086] 3. Adhesive - 70% ethanol solution appropriate amount
[0087] 4. Coating material—— E100 60.0g;
[0088] NE30D (dry) 5.0g;
[0089] 5. Filling agent - mannitol 400.0g;
[0090] Microcrystalline cellulose 120.0g;
[0091] 6. Flavoring agent—aspartame 5.0g;
[0092] Orange essence 5.0g;
[0093] 7. Disintegrant—cross-linked polyvinylpyrrolidone 120.0g;
[0094] Low-substituted hydroxypropyl cellulose 80.0g;
[0095] 9. Lubricant—magnesium stearate 10.0g.
[0096]
[0097] A total of 10,000 pieces were made
[0098] 2. Preparation method
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com