Synthesizing process and use of unsaturation cyclic aliphatic carbonate monomer and its polymer
A technology of cyclic aliphatic and aliphatic carbonates, which is applied in organic chemistry and other fields, and can solve the problems of complex synthesis process and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
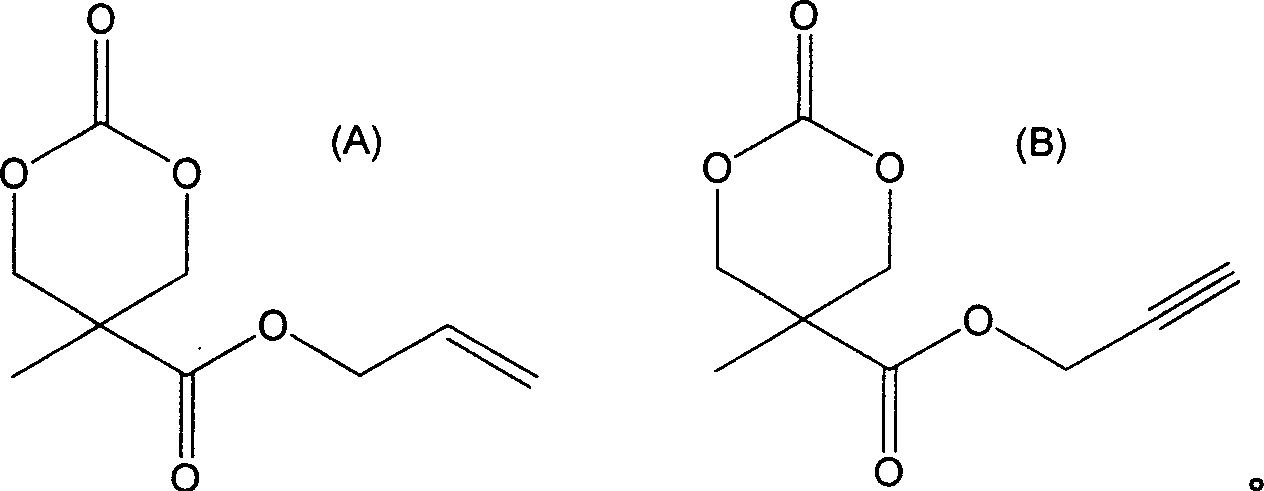
Image
Examples
Embodiment 1
[0058] Example 1: Synthesis of 2-methyl-2-allyloxycarbonyl-1,3-propanediol
[0059] 9.00 g of 2,2-dimethylolpropionic acid and 4.30 g of potassium hydroxide were dissolved in 50 ml of N,N-dimethylformamide. Stir vigorously for 1 h at 100°C to form potassium salt of 2,2-dimethylolpropionate. Propylene bromide was then added to the above solution, and stirred vigorously for 15 h at 100°C. The solvent was evaporated, the residue was dissolved in 200ml of ether, washed three times with 50ml of distilled water, and finally recrystallized with toluene to obtain 7.9g of 2-methyl-2-allyloxycarbonyl-1,3-propanediol with a yield of 68%. Its structure was confirmed by proton NMR spectroscopy.
Embodiment 2
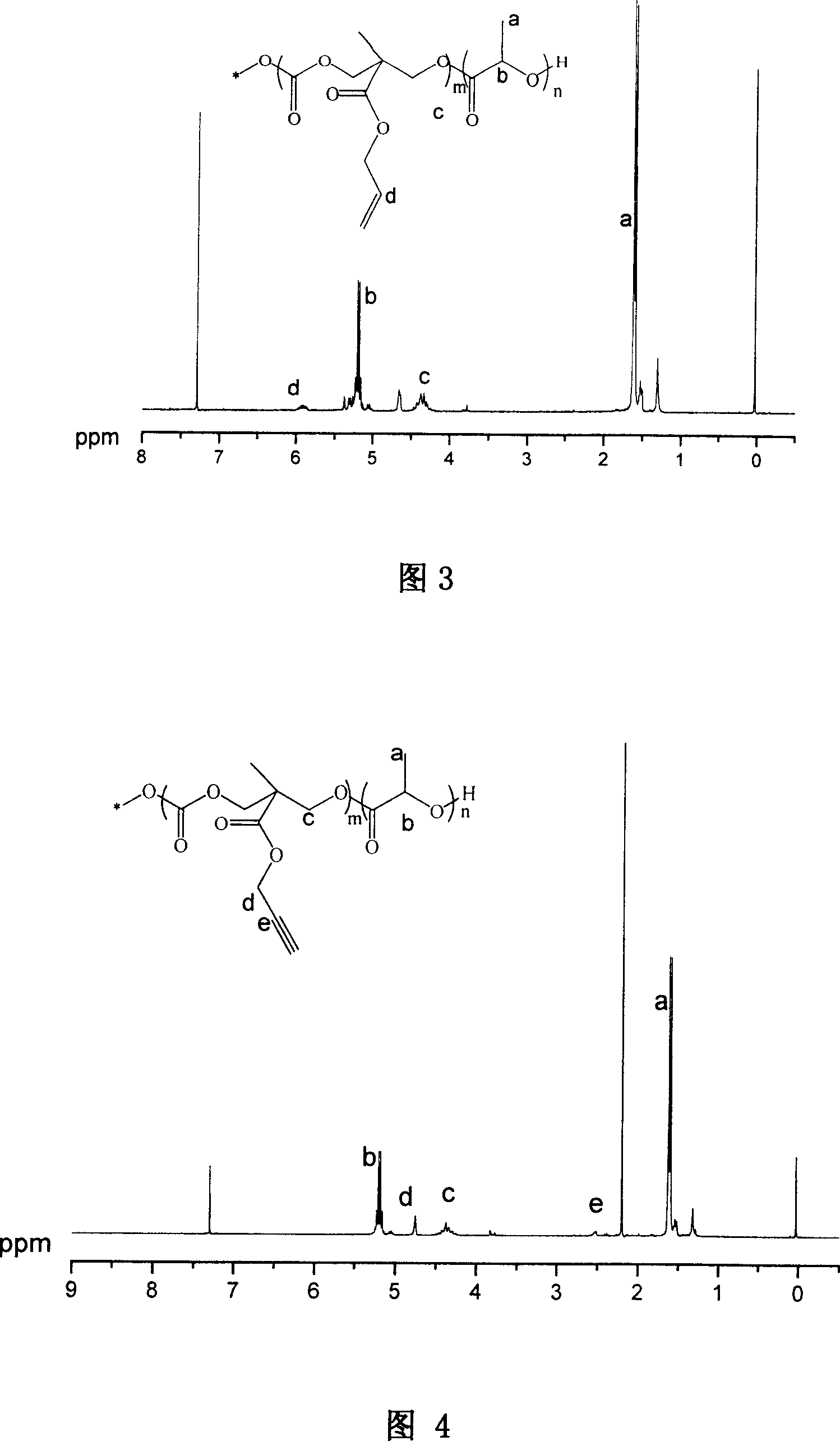
[0060] Example 2: Synthesis of 2-methyl-2-propargyloxycarbonyl-1,3-propanediol
[0061] 9.00 g of 2,2-dimethylolpropionic acid and 4.30 g of potassium hydroxide were dissolved in 50 ml of N,N-dimethylformamide. Stir vigorously for 1 h at 100°C to form potassium salt of 2,2-dimethylolpropionate. Then propargyl bromide was added to the above solution, and stirred vigorously at 100°C for 15h. The solvent was distilled off, the residue was dissolved in 200 ml of ether, washed three times with 50 ml of distilled water, and finally recrystallized with toluene to obtain 8 g of 2-methyl-2-propargyloxycarbonyl-1,3-propanediol with a yield of 70%. Its structure was confirmed by proton NMR spectroscopy.
Embodiment 3
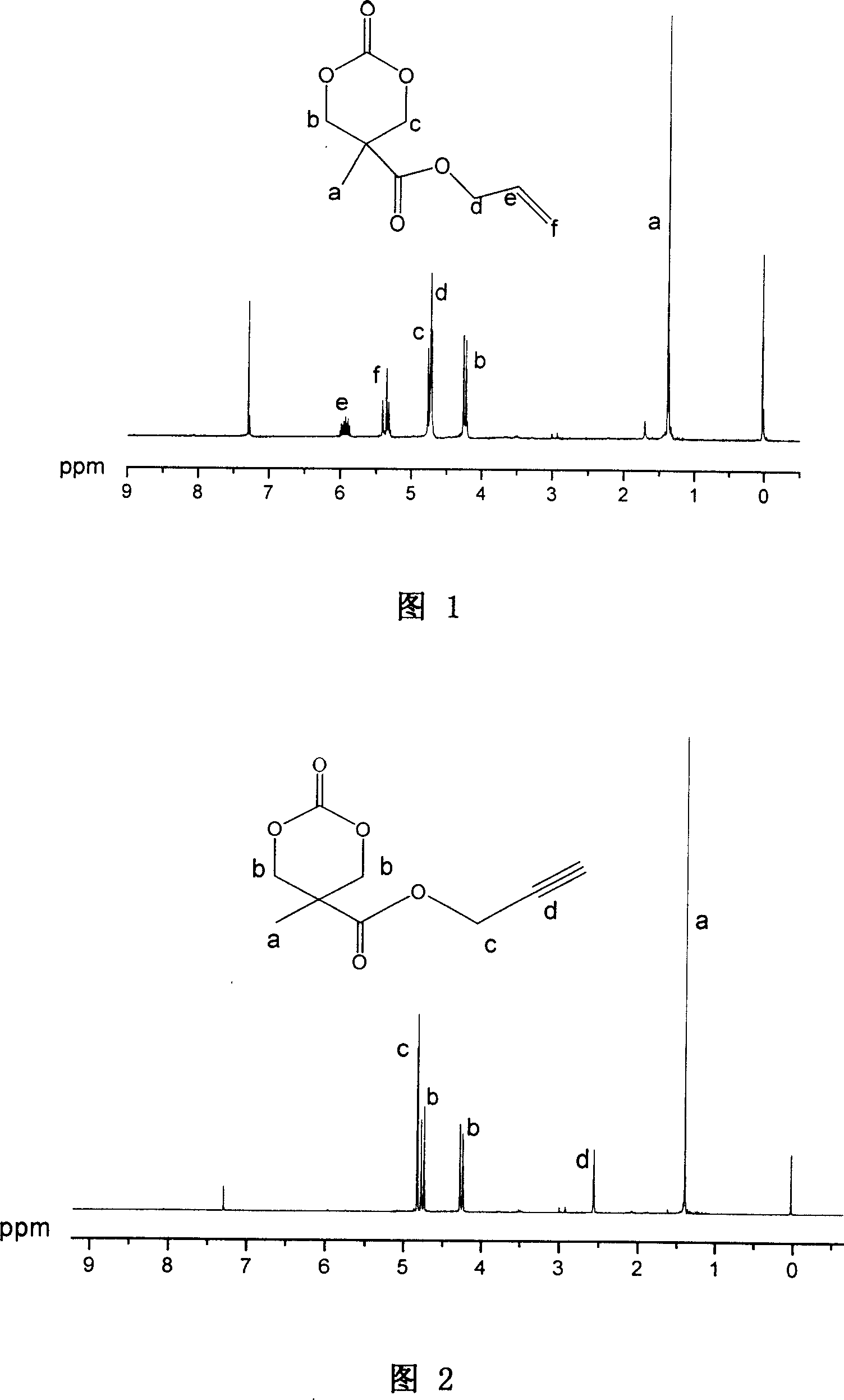
[0062] Embodiment 3: the synthesis of 2-methyl-2-allyloxycarbonyl trimethylene carbonate (monomer A)
[0063] Dissolve 10 g of 2-methyl-2-allyloxycarbonyl-1,3-propanediol and 28.5 g of ethyl chloroformate in 600 ml of tetrahydrofuran, and cool in an ice-water bath. Then slowly add 28g of triethylamine into the above solution, and keep the system at about 0°C during the addition. Then react at room temperature for 10h. The precipitate was filtered off, the filtrate was concentrated under reduced pressure, and the residue was recrystallized from tetrahydrofuran and diethyl ether to obtain 9.2 g of white crystals with a yield of 80%. Its structure is confirmed by proton nuclear magnetic resonance spectrum, see accompanying drawing 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com