Immuno-modulatory agents with function of preventing and curing type 2 diabetes
An immunomodulator, a technology for type 2 diabetes, which is applied in the field of immunomodulators and can solve problems such as large toxic side effects and inconvenience in use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Design, synthesis and cloning of Hsp65 polypeptide gene
[0052] Genomic DNA was extracted from the commercially available BCG vaccine (refer to the "Experimental Guide for Molecular Biology" for specific steps), and the entire gene of Hsp65 in the BCG vaccine was found by the National Center for Biotechnology Information (NCBI). On the premise of not changing the amino acid sequence of Hsp65, the preferred codons of E. coli were selected, and two primers H1 and H2 were designed with the aid of computer software.
[0053] The nucleotide sequences of the two primers are as follows:
[0054] H1: 5’TTCG CCATGG CCAAGACAATTGCGTACG 3’
[0055] H2: 5’TTCCG AAGCTT AGAAATCCATGCCACCCATG 3’
[0056] The upstream primer H1 introduced the Nco I restriction site, and the downstream primer H2 introduced the Hind III restriction site.
[0057] The PCR reaction system is: 100pmol H1, 100pmol H2, 2μg BCG genomic DNA, 2μldNTP (10mM), 5μl 10×Pfu buffer and 5 units of Pfu DNA polyme...
Embodiment 2
[0059] Example 2: Expression of Hsp65 gene in E. coli
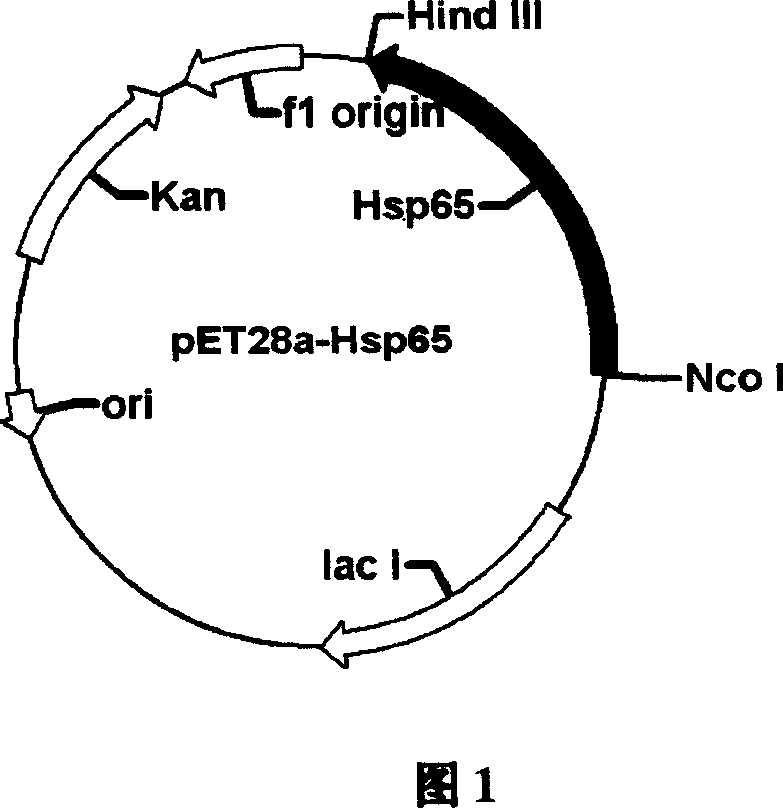
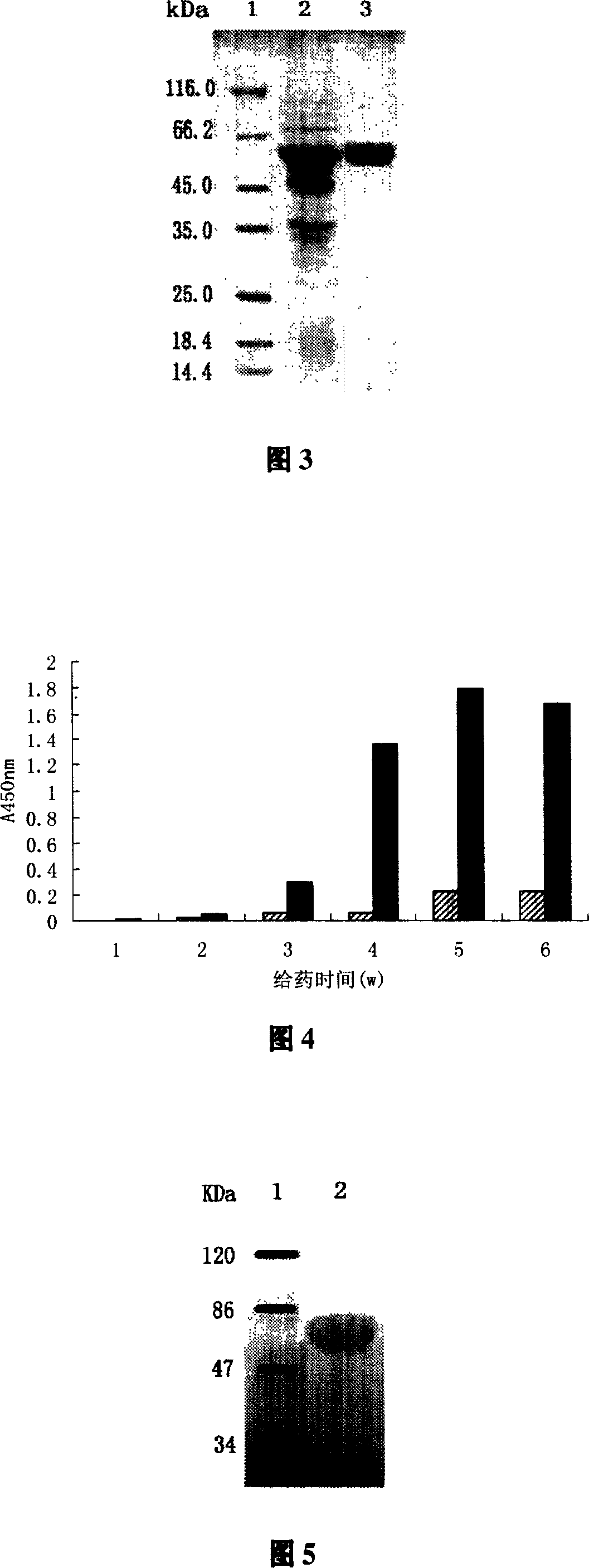
[0060] Pick a single colony from the pET28a-HspP65 plate to inoculate LB liquid medium containing 50μg / ml kanamycin, culture it overnight at 37°C with constant temperature shaking, and inoculate it into fresh corn steep liquor liquid medium (50μg / ml) at a rate of 1%. ml kanamycin), after culturing at 37°C for 4 hours, α-lactose at a final concentration of 5mmol / L was added to induce E. coli to express T7 RNA polymerase, and the culture continued to express protein Hsp65. After induction, a small amount of bacterial liquid was taken every 1 hour, and the bacterial cells were recovered by centrifugation. SDS-PAGE electrophoresis showed that the Hsp65 gene expression had been achieved. The protein reached a stable maximum expression level 6 hours after induction, and the protein accounted for about 40% of the total bacterial protein. The results are shown in Figure 3.
Embodiment 3
[0061] Example 3: Separation and purification of recombinant protein Hsp65
[0062] The engineered bacteria after induced expression were recovered by centrifugation, and the bacteria were suspended in the lysate (pH8.0, 50mmol / L phosphate buffer, 0.02% lysozyme), stirred at 37°C for 30 minutes, and added DNase I The DNA is digested until the solution is not viscous, the supernatant is recovered by centrifugation, and fractionated and precipitated with ammonium sulfate. The target protein is mainly in the 35%-45% ammonium sulfate precipitation. Re-dissolve the precipitated protein in ion exchange buffer (10mmol / L PB, pH6.8), dialysis desalting, centrifuge and take the supernatant for anion exchange column chromatography (DEAE cellulose DE-52 column, 2×60cm) , Use ion exchange buffer (10mmol / L PB, pH6.8) / NaCl gradient elution, collect in sections for SDS-PAGE electrophoresis detection, Hsp65 is in the elution peak at 80-120mmol / L NaCl. The purity of the prepared samples was checked...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com