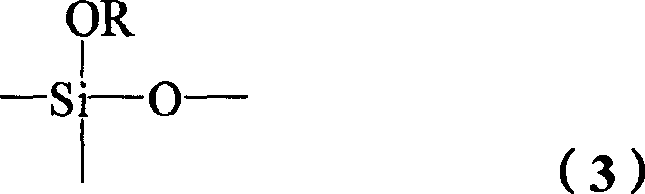Photosensitive resin composition
A technology of photosensitive resin and composition, which can be used in optics, optomechanical equipment, nonlinear optics, etc., and can solve problems such as deterioration of alkali developability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
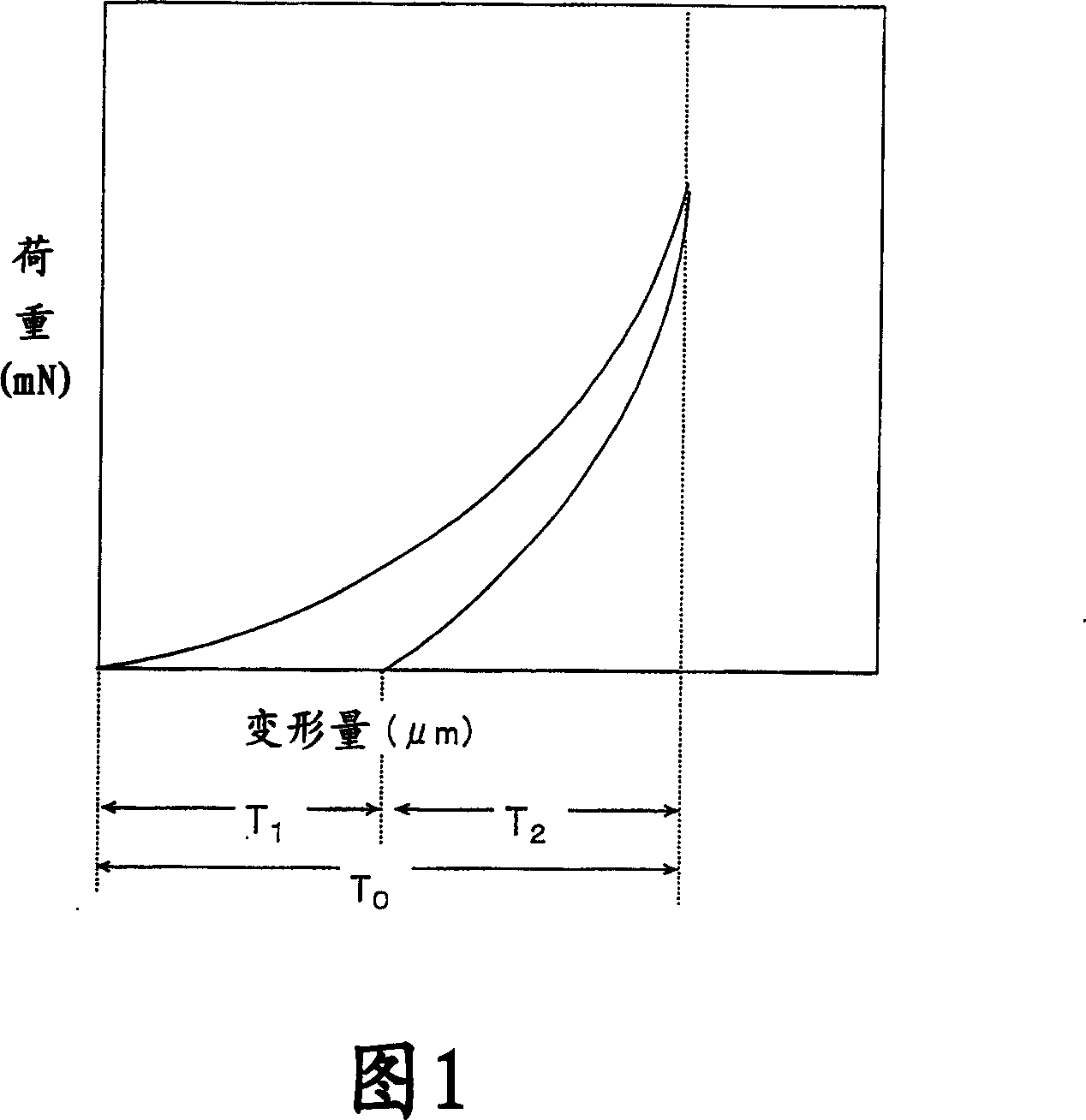
Image
Examples
preparation example Construction
[0097] The polycarboxylic acid and polycarboxylic acid anhydride (C) used in the preparation of (A2) can enumerate: unsaturated polycarboxylic acid and their anhydrides in above-mentioned (a), and saturated polyhydric (2-6 yuan) carboxylic acid ( For example, aliphatic saturated polycarboxylic acids such as oxalic acid, succinic acid, phthalic acid, adipic acid, lauric acid, dodecenyl succinic acid, pentadecyl succinic acid and octadecyl succinic acid; Aromatic polycarboxylic acids such as phthalic acid, hexahydrophthalic acid, methyl tetrahydrophthalic acid, trimellitic acid, pyromellitic acid, biphenyl tetracarboxylic acid and naphthalene tetracarboxylic acid) and their Anhydrides (e.g. aliphatic saturated polycarboxylic anhydrides such as succinic anhydride, dodecenyl succinic anhydride, pentadecenyl succinic anhydride and octadecenyl succinic anhydride; phthalic anhydride, tetrahydrophthalic anhydride, hexahydro Aromatic polycarboxylic acid anhydrides such as phthalic anhy...
Embodiment
[0236] Hereinafter, the present invention will be specifically described by way of examples and preparation examples, but the present invention is not limited thereto. Hereinafter, "part" means "part by weight".
[0237] [Preparation of Hydrophilic Polymer (A)]
preparation example 1
[0239] 50 parts (33 mol%) of isobornyl methacrylate, 30 parts (33 mol%) of methacrylic acid 2 -Hydroxyethyl ester, 20 parts (34 mole %) of methacrylic acid and 150 parts of cyclohexanone, heated to 80°C. After the gas phase in the system was replaced with nitrogen, 55 parts of azobisisobutyronitrile (V-60: manufactured by Wako Pure Chemical Industries, hereinafter referred to as AIBN) was dissolved in 50 parts of cyclohexanone, and then reacted at the same temperature for 3 hours to obtain a hydrophilic polymer (A-1) with hydroxyl and carboxyl groups (Mn: 8,800, SP value: 11.86, HLB value : 11.98, weak acid value: 102mgKOH / g) cyclohexanone solution (solid content: 25%). It should be noted that Mn is determined by GPC using a GPC analyzer (HLC-8120GPC, manufactured by Tosoh Corporation) and a chromatographic column (2 pieces of TSKgelGMHXL+TSKgel Multipore HXL-M, manufactured by Tosoh Corporation). The value measured in terms of polystyrene. SP value, HLB value and weak acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com