Iron composite/halogen electrochemical system for flow electric storage
A complex and electrochemical technology, applied in the field of iron-complex/halogen new electrochemical system, can solve the problems of large energy loss, hydrogen evolution side reaction, slow reaction kinetics, etc., and achieve high energy conversion efficiency and low preparation cost , long service life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh an appropriate amount of Fe 2 (SO 4 ) 3 and ligand-EDTA, the molar ratio of the two is 0.5 (0.5mol Fe 2 (SO 4 ) 3 Equivalent to 1molFe 3+ ion), dissolved in 1M sodium acetate aqueous solution, dissolved by magnetic stirring at room temperature, and then made into 0.1M Fe(III)-EDTA complex aqueous solution, and the pH value of the solution was maintained at about 5.5. An appropriate amount of NaBr was weighed and dissolved in water, and dissolved by magnetic stirring at room temperature to prepare a 1M NaBr aqueous solution.
[0026] Nafion117 membrane (DuPont, USA) was selected as the cation exchange membrane separating the positive / negative electrolyte. The membrane needs to be treated as follows before use: heat treatment in a water bath of 353K in a 1.0mol / L NaOH aqueous solution for 2h, then, use the After washing with ionized water, the hydrogen-type membrane is converted into a Na-type membrane, which can be used in batteries.
[0027] The prepared po...
Embodiment 2
[0030] The raw material Fe used in the preparation of negative electrolyte 2 (SO4) 3 With embodiment 1: take by weighing an appropriate amount of Fe 2 (SO4) 3 and ligand-sodium citrate, the molar ratio of the two is 0.5, dissolved in 1M sodium acetate aqueous solution, dissolved by magnetic stirring at room temperature, and made into 0.7M Fe(III)-citric acid complex aqueous solution. The pH value remains between 5-6. The prepared negative electrolyte was stored for later use.
[0031] The preparation of the positive electrode electrolyte is the same as that in Example 1: Weigh an appropriate amount of NaBr and dissolve it in water, and after magnetic stirring at room temperature to dissolve, it is made into a 2M NaBr aqueous solution.
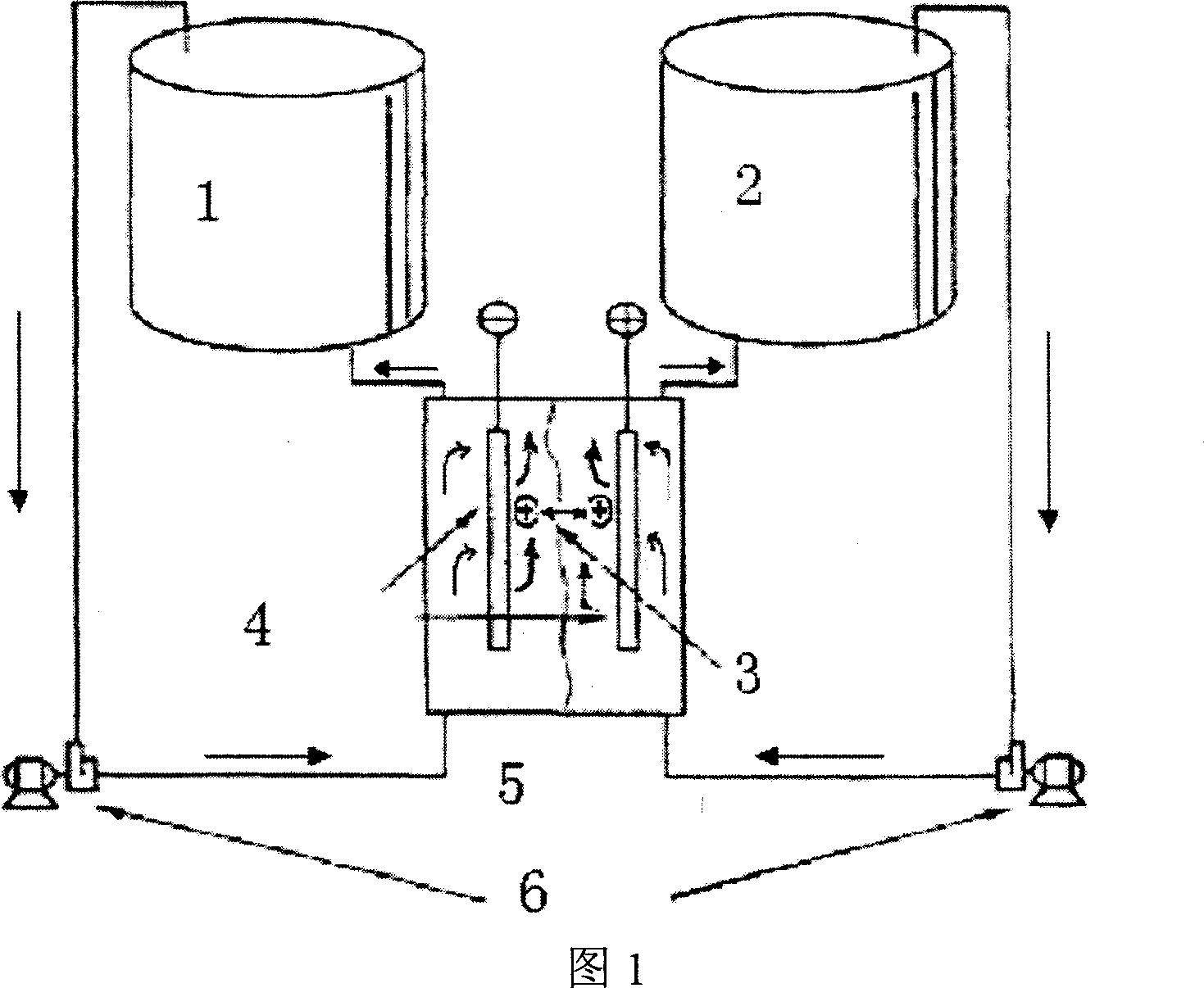
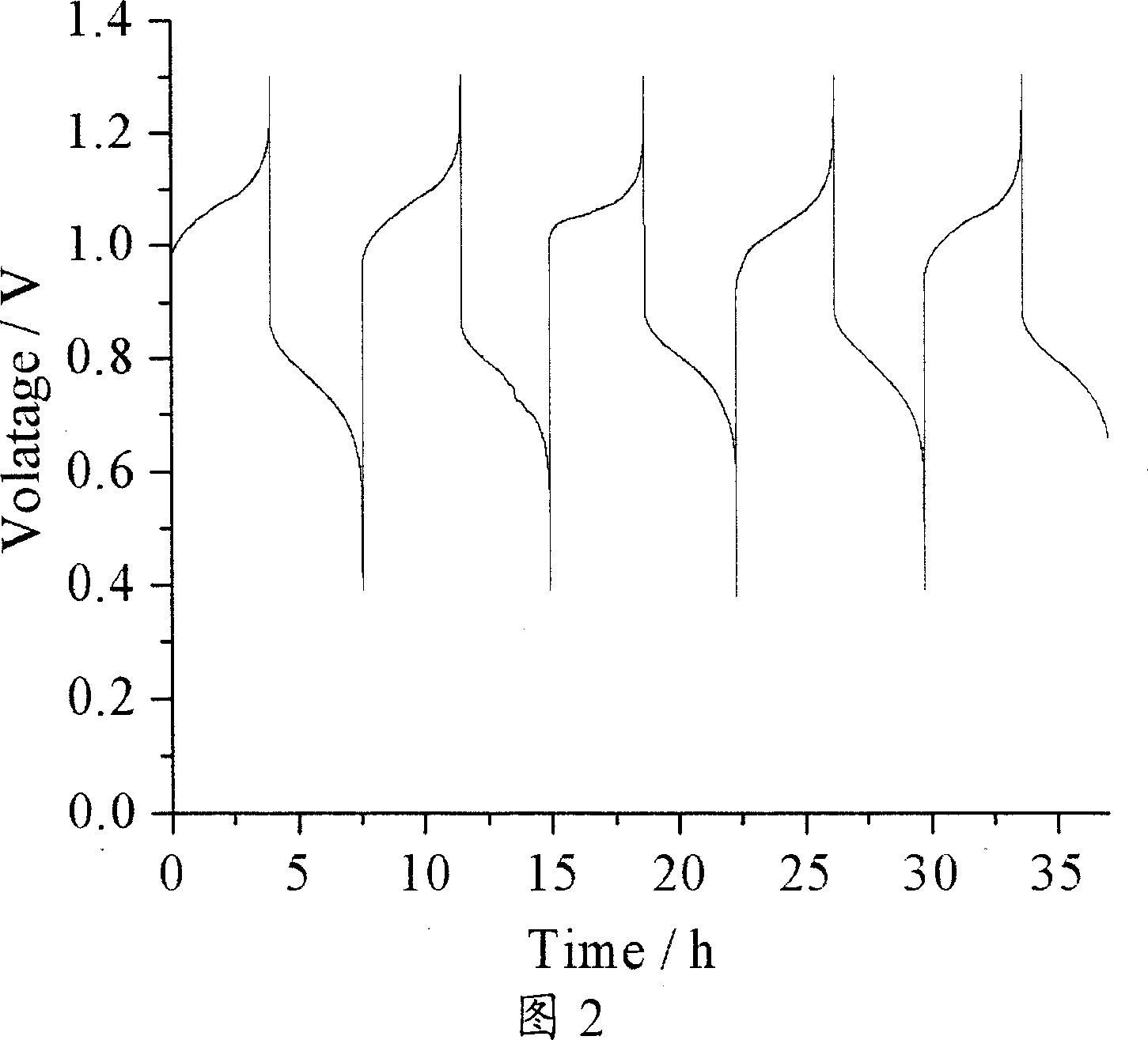
[0032] 50 mL of the prepared positive / negative electrolyte were placed in the peripheral liquid storage bottles on both sides of the battery, and assembled into a battery as shown in Figure 1. The operating conditions of the battery are th...
Embodiment 3
[0034] The raw material Fe used in the preparation of negative electrolyte 2 (SO 4 ) 3 With Example 1, weigh an appropriate amount of ligand-triethanolamine (TEA) and Fe 2 (SO 4 ) 3 , the molar ratio of the two is 3. After mixing, add an appropriate amount of NaOH to adjust the pH value of the solution to about 13. At room temperature, magnetically stir, dissolve in 0.4M sodium chloride supporting electrolyte aqueous solution, and prepare 0.4M Fe (III)-TEA complex aqueous solution. The prepared negative electrolyte was stored for later use.
[0035] The preparation of the positive electrode electrolyte is the same as that in Example 1: Weigh an appropriate amount of NaBr and dissolve it in water, and after magnetic stirring at room temperature to dissolve, it is made into a 2M NaBr aqueous solution.
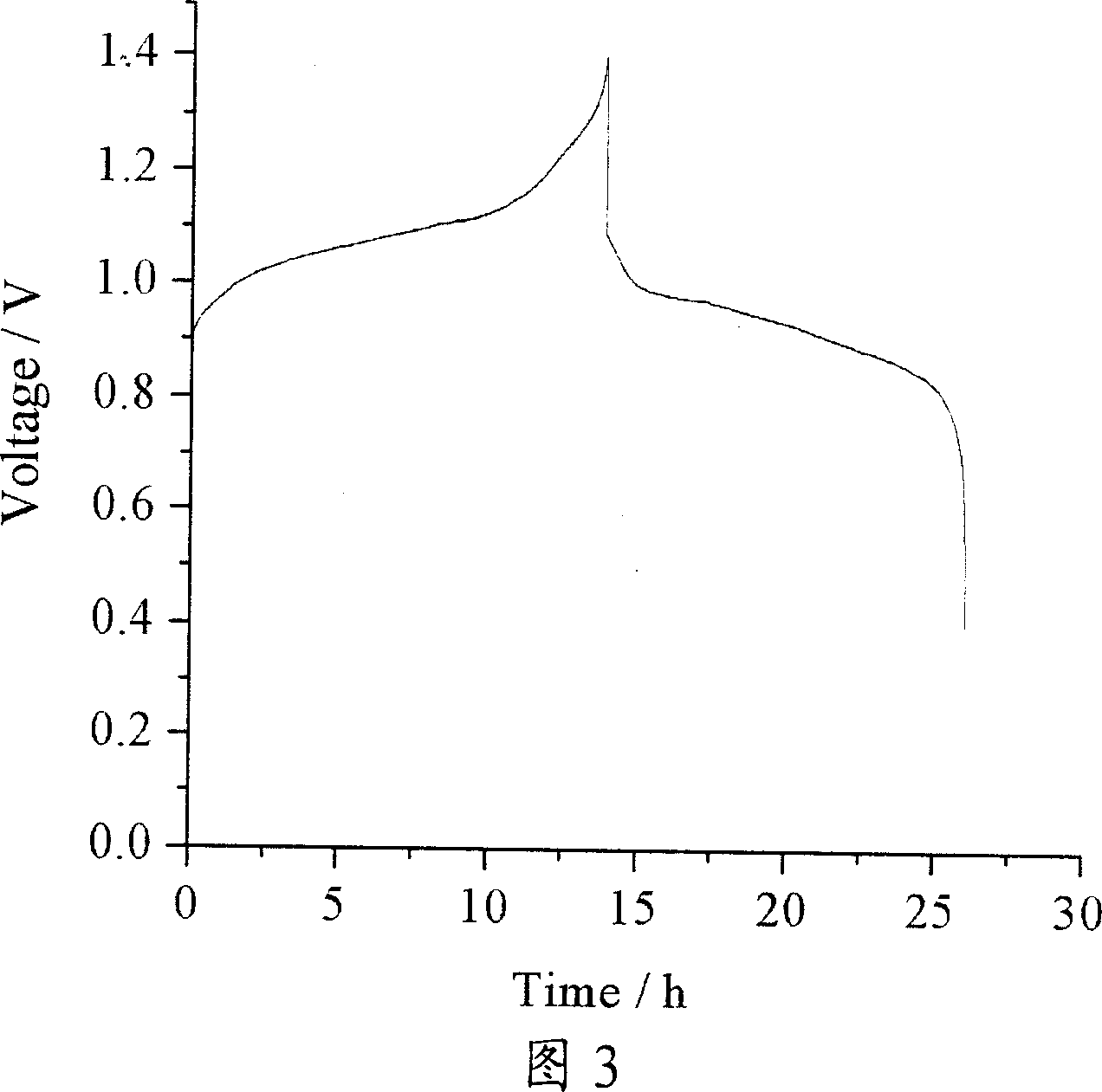
[0036] 50 mL of the prepared positive / negative electrolyte were placed in the peripheral liquid storage bottles on both sides of the battery, and assembled into a battery a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com