Lipopolysaccharide conjugated protein and monoclonal antibody and usage
A technology for binding protein and lipopolysaccharide, applied in the direction of antibody, anti-animal/human immunoglobulin, application, etc., can solve the problems of accelerated lipopolysaccharide binding protein gene, body damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Preparation and identification of the amino-terminal fragment of human lipopolysaccharide-binding protein:
[0096] (11) Obtain the cDNA sequence encoding human NH-LBP, and construct the expression virus:
[0097] Messenger RNA (mRNA) was extracted from hepatocytes of patients who died of infectious shock, reverse-transcribed into a cDNA library, and the double encoding human NH-LBP was amplified with specific primers 5′-GTCGACTGGAGTGGGAATCTAGGA-3′; 5′-AAGCTTATTCTGTGTTGTAACTGG-3′ Strand DNA sequence, connected with pMD19-T Simple Vector, transferred into competent DH-5a bacteria, double enzyme digestion with Sal I and HindIII after the test was correct, the target sequence was recovered, purified, and treated with the same enzyme as the vector pFASTBAC Ligated to construct plasmid pFASTBAC-NH-LBP. Transfer pFASTBAC-NH-LBP into DH10BAC bacteria, and construct the shuttle plasmid pBacmid-NH-LBP through gene translocation. After the sequencing is correct, pBacm...
Embodiment 2
[0141] Example 2: Preparation of Human Phage Antibody Library
[0142] Mononuclear cells were isolated from the peripheral blood of healthy volunteers, and the DNA sequence was extracted from the monocytes. Seven pairs of primers were used to amplify the variable region sequence (Fd) encoding antibody heavy chain IgVH, and four pairs of primers were used to amplify the sequence encoding The variable region sequence of antibody light chain IgVκ and 5 pairs of primers were used to amplify the variable region sequence of antibody light chain IgVλ, digested with Sac I+Xba I and Xho I+Spe I respectively, and pComb3H phagemid was used as The human phage antibody library Fab was established in XL1-Blue bacteria.
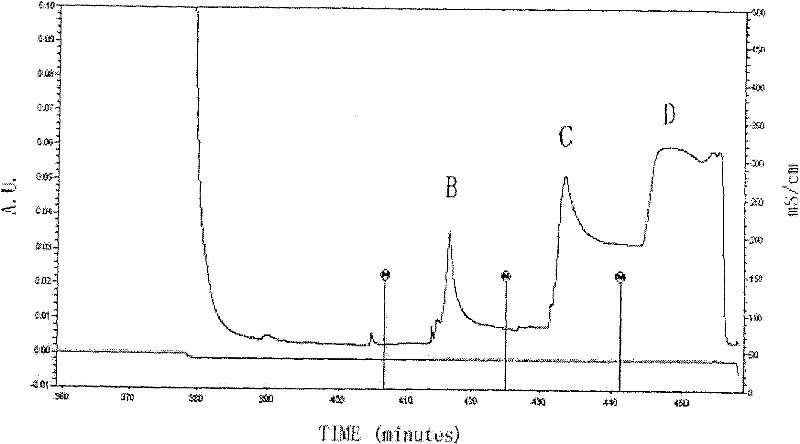
[0143] Such as Figure 4 Shown is the construction of the human phage antibody library and the results of enzyme digestion identification, in which:
[0144] 1: DNA markers (15000, 10000, 7500, 5000, 2500, 1000, 250bp);
[0145] 2: κbank containing pCOmb3H / Sac I+Xba I (c...
Embodiment 3
[0183] Example 3: Preparation of Human Anti-NH-LBP Monoclonal Fab Antibody and dsFv Antibody
[0184] (31) Using NH-LBP as an antigen to screen the Fab of the phage antibody library and identify a single strain:
[0185] Coat the enzyme-linked plate with NH-LBP as an antigen, add the phage antibody library after washing the plate, wash the plate after 2 hours of binding, wash off the bound phage, re-infect XL1-Blue bacteria and amplify the antibody library, and the amplified phage antibody The library was screened for the next round, with a total of 5 rounds of screening.
[0186] After 5 rounds of screening, the phages carrying anti-NH-LBP antibody were significantly enriched, and the binding ability was increased by 1.65×10 4 times, proving that the screening was successful.
[0187] Each single colony was amplified, induced by Isopropylβ-D-1-thiogalactopyranoside (IPTG), secreted and expressed Fab antibody, labeled with NH-LBP, and Fab was used as a Anti-HRP-mouse anti-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com