Biodegradation polymer and its preparing process and application
A biodegradable polymer technology, applied in pharmaceutical formulation, transportation and packaging, packaging items, etc., can solve the problems of lower glass transition temperature and shortened storage period of polymers at room temperature, so as to reduce demand, speed up degradation time, The effect of increasing Tg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] (a) step In the preparation of polyglycol ester, the most commonly used general reaction is according to the ring-opening esterification polymerization reaction between the diol of following formula and lactide:
[0130]
[0131] The advantage of the melt polycondensation reaction is that it avoids the use of solvents and a large number of other additives, which results in more convenient purification. It also yields reasonably high molecular weight polymers. However, more severe conditions are usually required to make the chain acidolyzable (or hydrolyzable in the presence of water). If the polymer backbone is susceptible to deprivation of hydrogen atoms or oxidation due to subsequent recombination of large free radicals, undesired, thermally induced side reactions, such as crosslinking reactions, may occur.
[0132] The function of the first step reaction (a) is to use an initiator to open the ring of the heterocyclic compound of structural formula III, IV or V. ...
Embodiment 1
[0187] Embodiment 1: Taking 1,4-cyclohexanedimethanol (CHDM) as starting material synthesis poly(L-lactide-cyclohexanedimethanol-ethyl phosphate LAEG-CHDM-EOP) polymer
[0188] 20 g (0.139 mol) of (3S)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione (L-lactide LAEG) (purchased from Aldrich Chemical Company , recrystallized from ethyl acetate, sublimed and recrystallized again from ethyl acetate) and 2.0 g (13.9 mmol) of 1,4-cyclohexanedimethanol were placed in a 250 ml round-bottomed flask filled with dry argon. The flask was sealed under vacuum and placed in an oven heated to 140°C. The flask was maintained at this temperature for about 48 hours with intermittent shaking.
[0189] Then take out the flask and lower it to room temperature, add 200ml of chloroform and stir to dissolve, put it into an ice-salt bath to cool down, and add 4ml of triethylamine. Under argon flow and stirring, 2.26 g of ethyl dichlorophosphate was slowly added while maintaining 20-30°C. After stirring f...
Embodiment 2
[0190] Example 2: Properties of PolyLAEG-CHDM-EOP Prepared as Described in Example 1
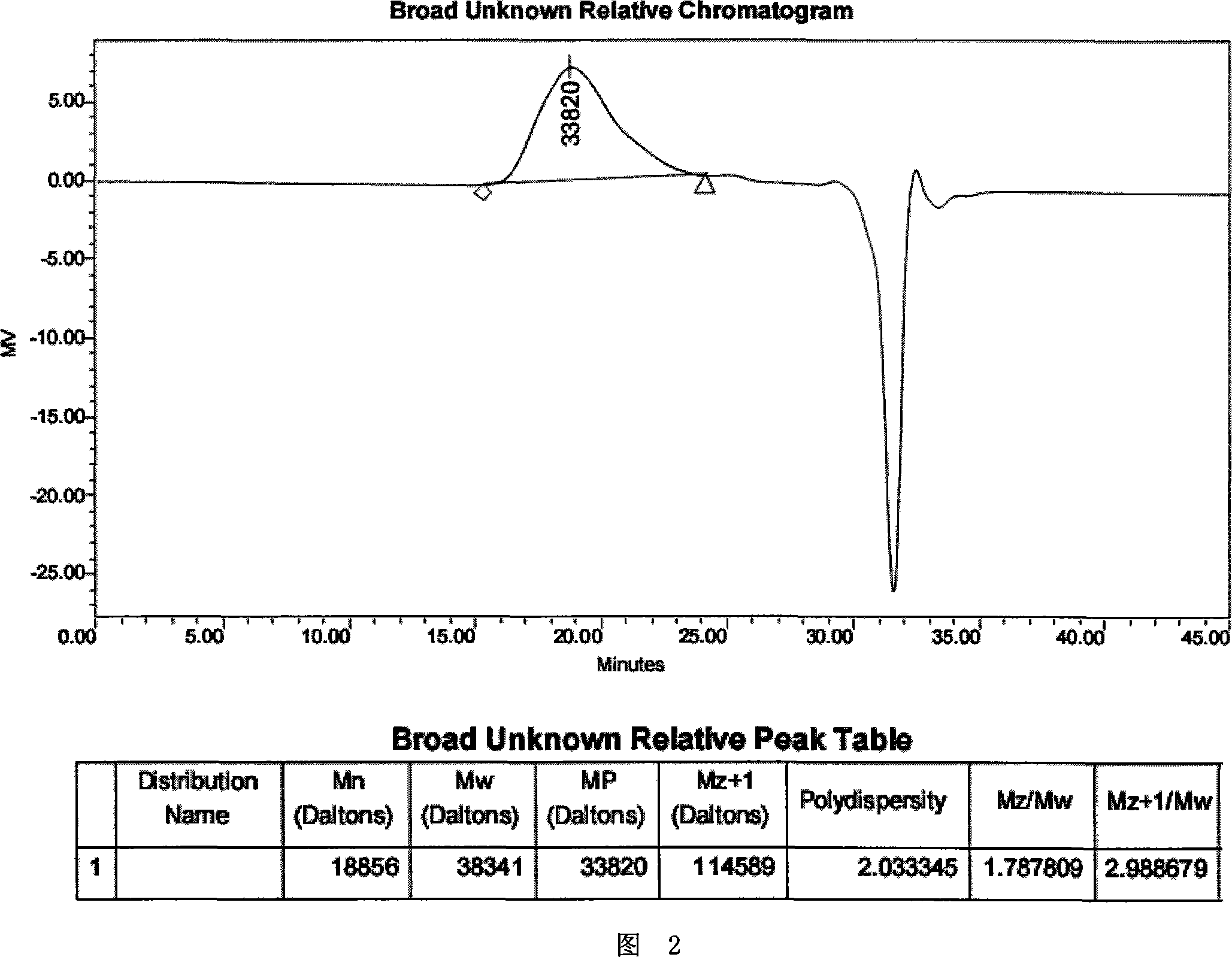
[0191] A polymer prepared as described in Example 1, wherein (x or y) / n=10:1. The resulting poly( LAEG-CHDM-EOP ) The polymer was analyzed by GPC using polystyrene as a standard, and the resulting figure showed that Mw was 38341 and Mn was 18856, as shown in Figure 2.
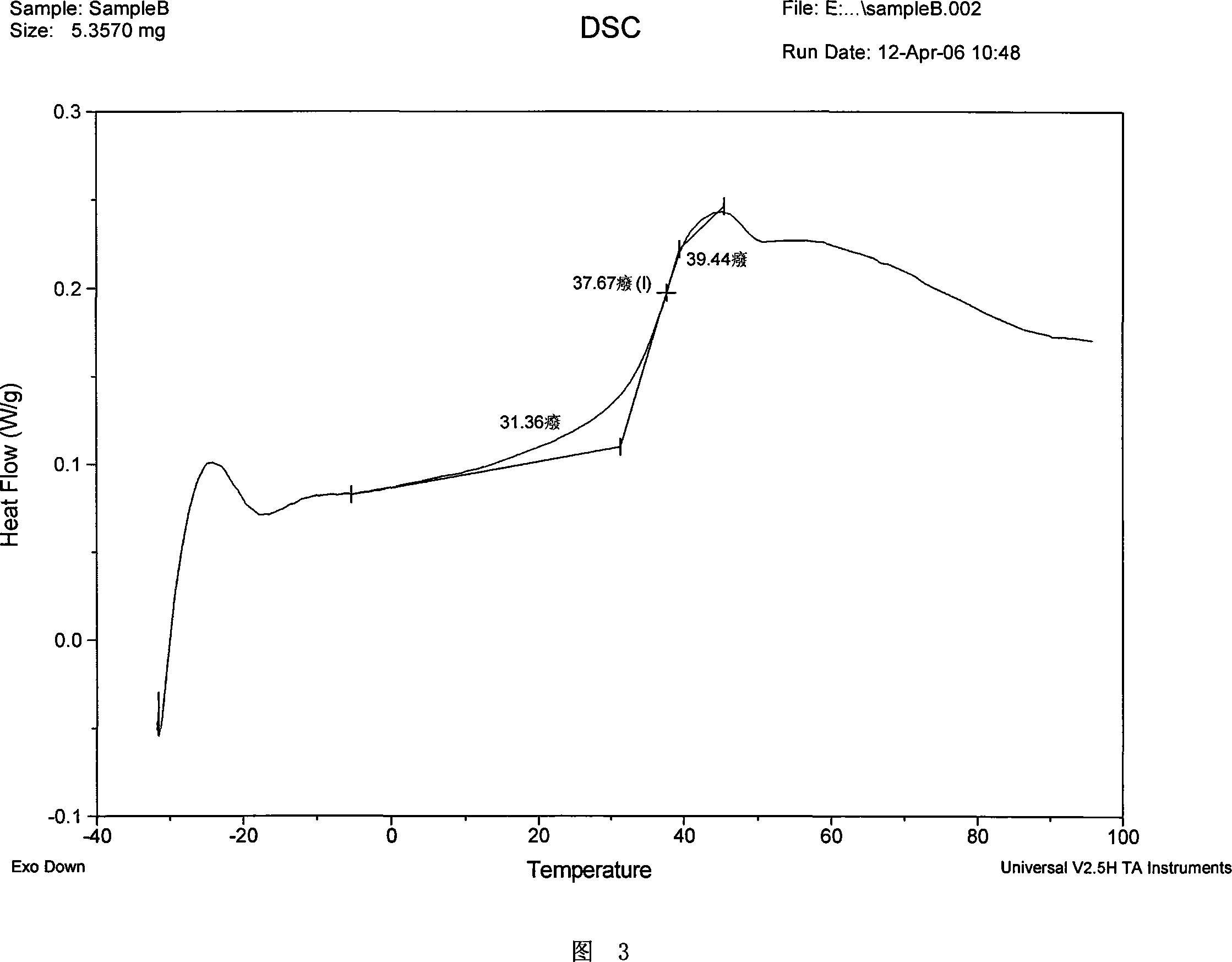
[0192] The Tg measured by DSC is 37.67° C., as shown in FIG. 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com