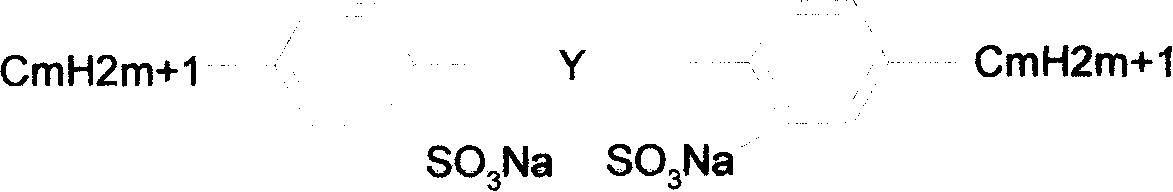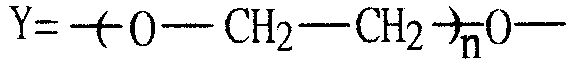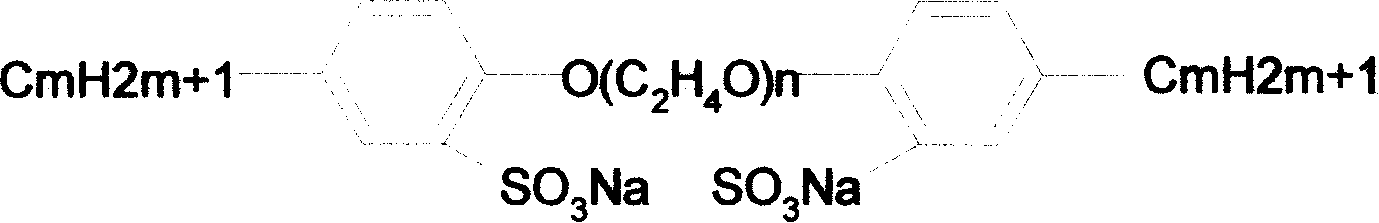Gemini anionic surface active agent and preparation method thereof
A technology of surfactants and anions, applied in chemical instruments and methods, dissolution, chemical/physical processes, etc., to achieve high surface activity, easy access to raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: In a 100ml four-necked flask, add 30ml of dichloroethane, 2g (0.015mol) AlCl 3 , Stir and heat to 60°C until it dissolves completely. Add 3.4g (0.02mol) of diphenyl ether, dropwise add 24.92g (0.1mol) of dodecane bromide, control the temperature at 75°C, and react for 6h. After the reaction is completed, wash with an equal volume of deionized water, saturated sodium carbonate solution, and deionized water successively, separate, discard the water layer, dry the oil layer with anhydrous sodium sulfate, suction filter, and distill under reduced pressure until the bromoalkane is completely After distilling off, 8.11 g of the remaining dark yellow liquid was obtained, which was dialkyl diphenyl ether, and the yield was 80.14%. Move to a 100ml four-neck flask, add 20ml of dichloroethane, dropwise add 4.2g (0.036mol) chlorosulfonic acid in an ice bath, after the dropwise addition is complete, raise the temperature to 35°C, and react for 2h. Neutralize with 15% Na...
example 2
[0037] Example 2: Put 22g (0.1mol) of nonylphenol in a 250ml four-necked flask, add 2g of cetyltrimethylammonium bromide and 100ml of 15% sodium hydroxide solution, stir, and heat to 70°C, drop Add 7.15g (0.05mol) of dichlorodiethyl ether, continue to heat up to 90°C, react for 2 hours, and stop the reaction. After the system cools down, add 50 mL of ether to the system for extraction three times, wash the extract with 3% acetic acid solution, wash with water, dry over anhydrous magnesium sulfate, filter, and then spin dry the solvent with a rotary evaporator to obtain 18.65 g of diethyl ether. Nonylphenol diethyl ether. Place bis-nonylphenol diethyl ether in a three-necked flask equipped with a hydrogen chloride absorption device, add 50 mL of anhydrous dichloromethane, stir, dissolve 4.26 g (0.037 mol) of chlorosulfonic acid in 30 mL of anhydrous dichloromethane, and Drop into the reaction system under ice bath, continue to react at 35°C for 2h, stop the reaction, neutraliz...
example 3
[0038] Example 3: the gemini type sulfonate surfactant didodecyl diphenyl ether sodium disulfonate prepared by example 1 has been tested for surface activity and application performance, and the test results are as follows:
[0039] At 25°C, the minimum surface tension of the aqueous surfactant solution is 39.1mN m -1 , with a critical micelle concentration of 6.32×10 -5 mol / L, the foam height is 360mm, the stability is up to 2h, the calcium soap dispersibility is 10.5, and the decomposition temperature is greater than 400℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Critical micelle concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com