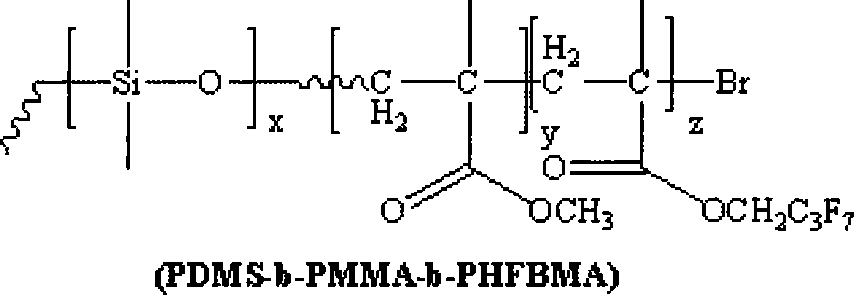
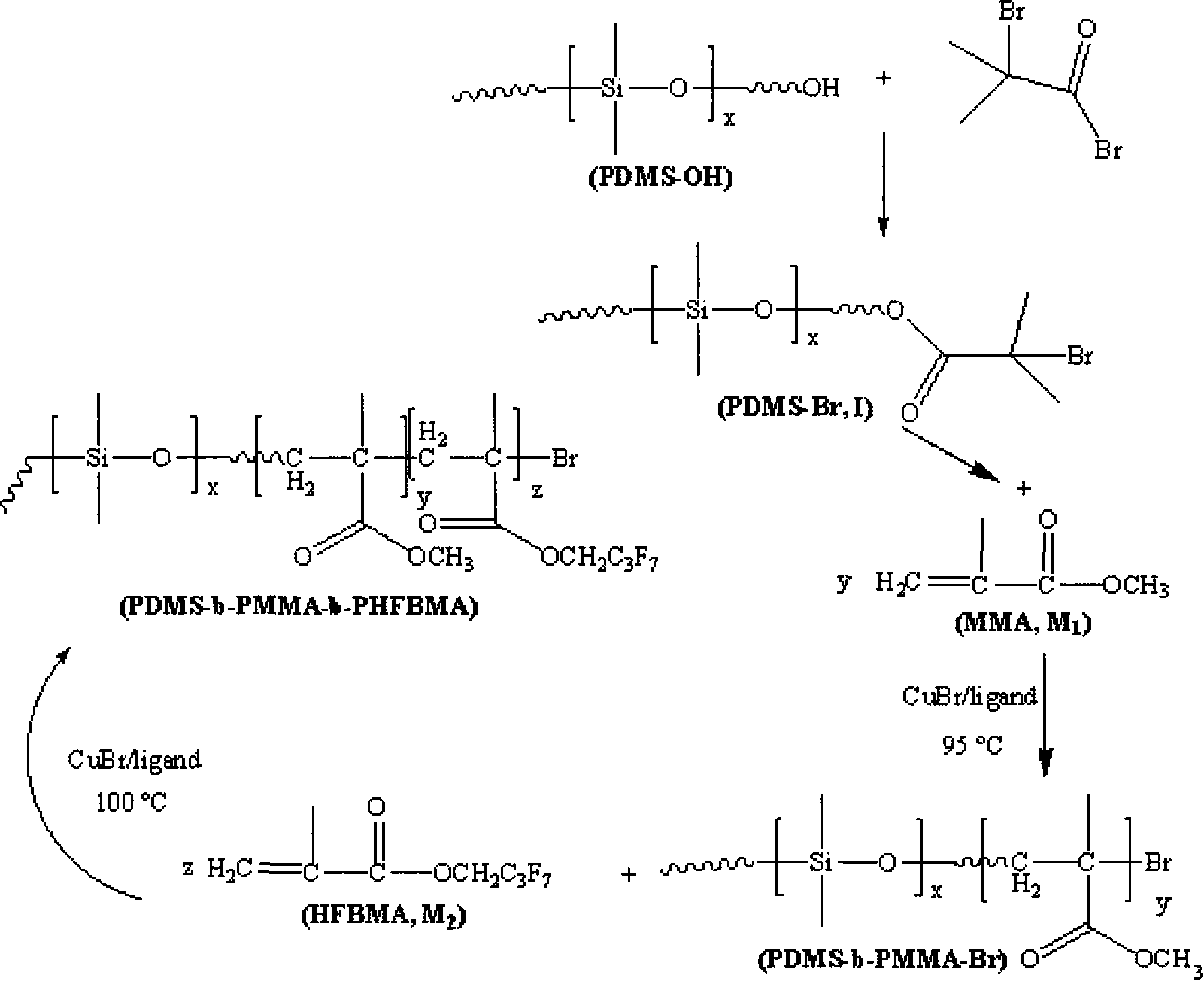
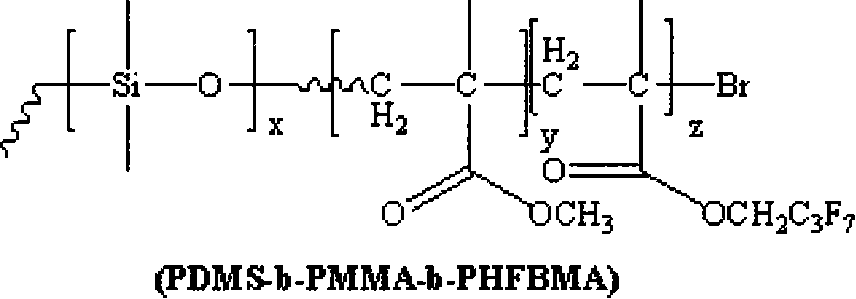
Fluorine silicon tri-block copolymers and preparation method thereof
The technology of block copolymer and triblock is applied in the field of fluorosilicon triblock copolymer and its preparation. The effect of narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Under the protection of nitrogen atmosphere, react 1 part of monomethanol-terminated polydimethylsiloxane, 1.2 parts of 2-bromoisobutyryl bromide and 2.5 parts of triethylamine at 5°C. Then the above solution was stirred and reacted for 30 hours. After the reaction was completed, it was filtered, and the filtrate was distilled off under reduced pressure to remove the solvent, and then dissolved in dichloromethane, washed several times with saturated sodium bicarbonate solution, separated, and the organic layer was Use anhydrous magnesium sulfate to dry and remove water, then filter, and finally the filtrate is distilled off under reduced pressure to remove the methylene chloride solvent to obtain an oily yellow macromolecular initiator;
[0022] 2) The reaction system must be strictly deoxygenated, using 1 part of macromolecule as initiator, 1 part of cuprous bromide as catalyst, and 2 parts of N-(n-propyl)-2-pyridinemethanamine as catalyst ligand React with 10 parts...
Embodiment 2
[0025]1) Under the protection of argon atmosphere, react 1 part of monomethanol-terminated polydimethylsiloxane, 1.5 parts of 2-bromoisobutyryl bromide and 2.8 parts of triethylamine at 10°C. Then the above solution was stirred and reacted for 20 hours. After the reaction was completed, it was filtered, and the filtrate was distilled off under reduced pressure to remove the solvent, and then dissolved in dichloromethane, washed several times with saturated sodium bicarbonate solution, separated, and the organic layer was Use anhydrous magnesium sulfate to dry and remove water, then filter, and finally the filtrate is distilled off under reduced pressure to remove the methylene chloride solvent to obtain an oily yellow macromolecular initiator;
[0026] 2) Except that the ligand is 1,1,4,7,7-pentamethyldivinyltriamine, the monomer is 20 parts, and the reaction temperature is 50° C., the others are the same as step 2 in Example 1);
[0027] 3) Except that the reaction temperatur...
Embodiment 3
[0029] 1) Except that 2-bromoisobutyryl bromide is 3.6 parts and triethylamine is 4.6 parts, other are the same as step 1 in Example 1);
[0030] 2) except that the monomer is 30 parts, other is the same as step 2 in the embodiment 2);
[0031] 3) Except that the reaction temperature is 70° C. and the ligand is 1,1,4,7,7-pentamethyldivinyltriamine, the procedure is the same as step 3) in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com