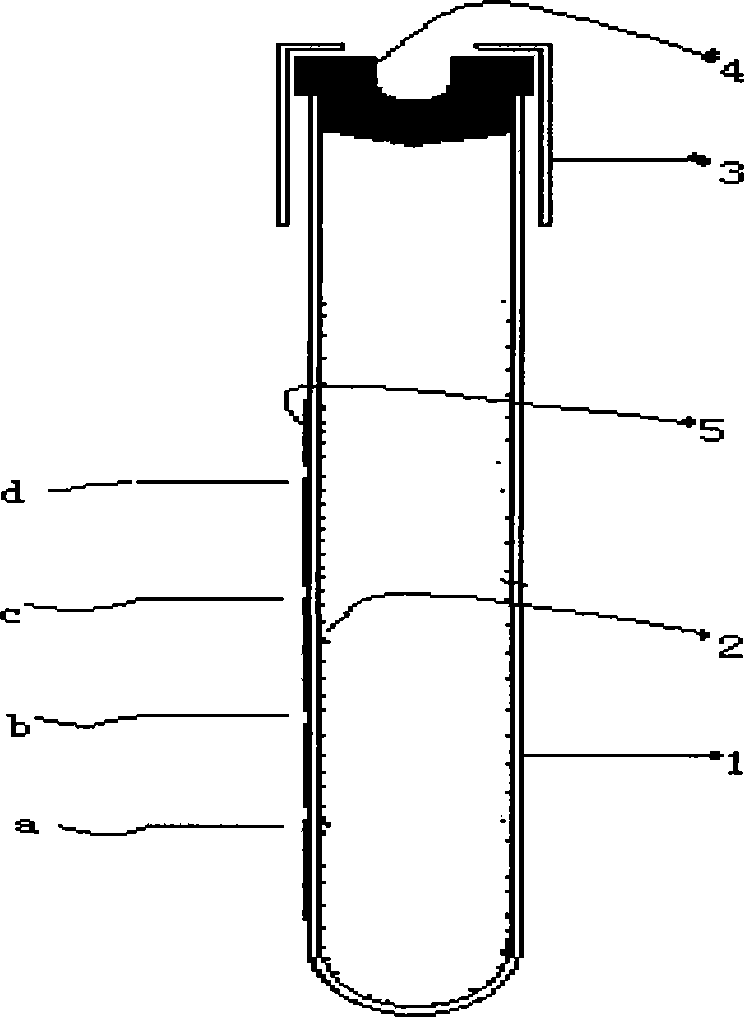
Vacuum hemostix
A vacuum blood collection tube, vacuum tube technology, applied in fungi, sensors, diagnosis and other directions, to achieve the effect of extending shelf life, large workload and improving work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the preparation of vacuum blood collection tube
[0018] 1. Preparation of Hirudin
[0019] a) Synthesis of Hirudin Gene
[0020] The DNA sequence shown in Sequence 2 in the sequence listing was synthesized by a fully artificial synthesis method. The two ends of the sequence have recognition sites for restriction endonucleases EcoRI and BamHI. The DNA shown in Sequence 2 is from the 5' end of the 262nd-459th position. bit encodes the protein of Sequence 1 in the Sequence Listing. The synthesized DNA sequences were digested with EcoRI and BamHI, and the digested fragments were recovered by electrophoresis, and stored at -20°C for later use.
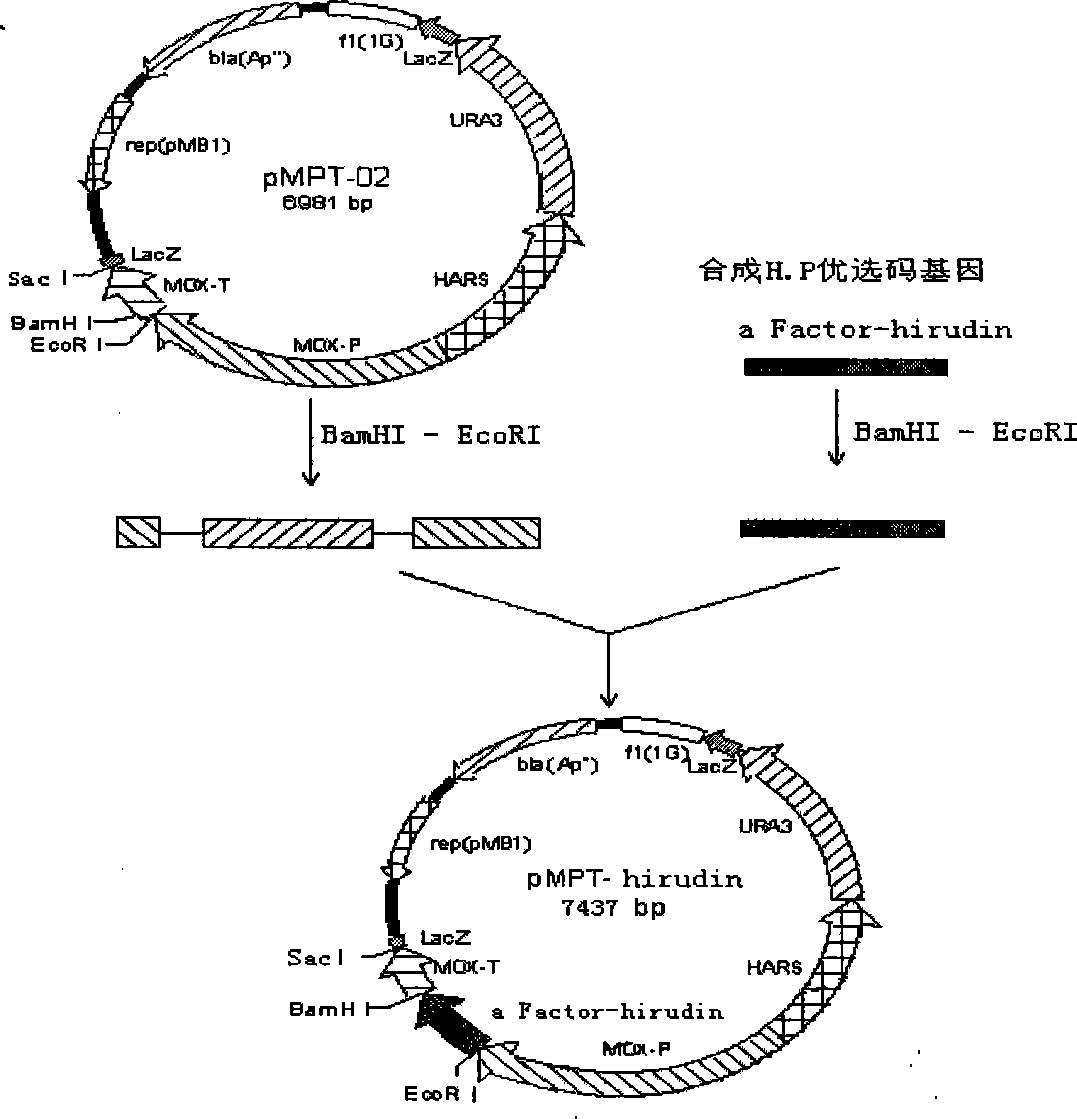
[0021] b) Construction of vector pMPT-02
[0022] Using Hansenula polymorpha CGMCC 2.2497 genomic DNA as a template, clone 1.5kb methanol oxidase gene (MOX) promoter, 350bp methanol oxidase gene (MOX) terminator, 1.0kb autonomously replicating sequence HARS; and cloned from YIp5 The 1.1kb Saccharomyces cerevisiae u...
Embodiment 2
[0108] Example 2. Stability test of anticoagulant activity of general vacuum blood collection tubes.
[0109] The prepared universal vacuum blood collection tubes were stored at 4°C and room temperature, respectively, and their anticoagulant activity was measured at different times. The method for measuring the anticoagulant activity of general vacuum blood collection tubes is basically the same as the method for measuring the anticoagulant specific activity of hirudin, except that the abscissa of the regression line equation is changed from the mass of hirudin to the inverse of the volume of the diluent. Table 1 shows the results of the three determinations of the stability test of the anticoagulant activity of the universal vacuum blood collection tube.
[0110] Table 1. Determination of Anticoagulant Activity Units of Recombinant Hirudin Universal Vacuum Blood Collection Tube
[0111] storage time
[0112] The above-mentioned universal vacuum blood co...
Embodiment 3
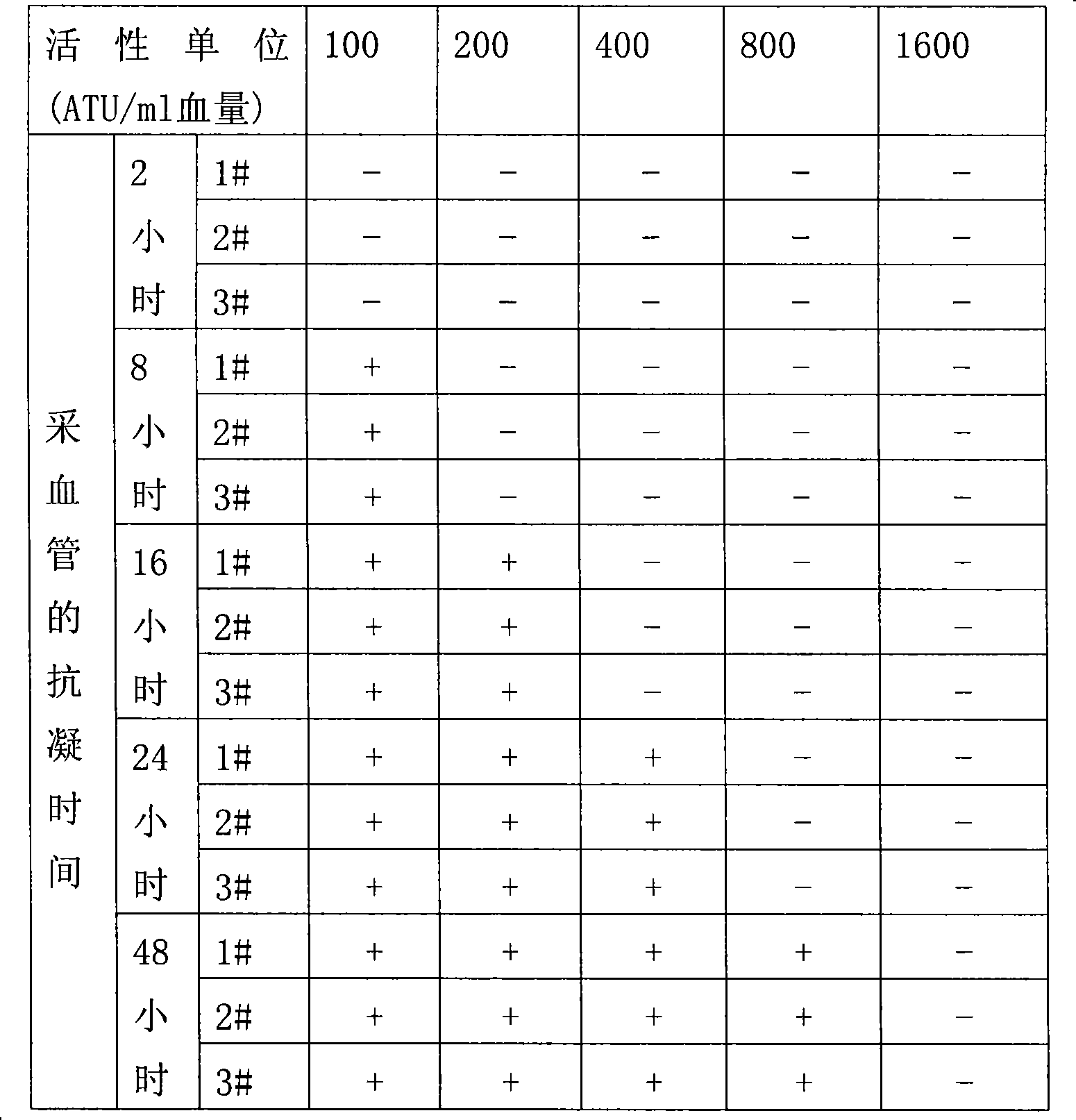
[0113] Example 3. Anticoagulation time of the universal vacuum blood collection tube of the present invention
[0114] The preset activity units of recombinant hirudin in blood collection tubes are 100, 200, 400, 800 and 1600 ATU / ml blood volume; three healthy subjects are named 1#, 2# and 3# respectively, and each person collects 5 tubes of blood , each tube of 2ml, the application of Japan Optoelectronics Celltac MEK-6318 automatic blood analyzer (white blood cells 5 classification), automatic detection of blood cells. There is an obvious dose-effect relationship between the different activity units of the preset recombinant hirudin and the anticoagulation time of the blood collection tube, that is, the higher the activity unit of the recombinant hirudin, the longer the anticoagulation time of the blood collection tube. The results are shown in Table 2:
[0115] Table 2. The relationship between the active unit of recombinant hirudin and the anticoagulation time of blood co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com