Isosorbide dinitrate oral administration impulse pellet preparations
A technology of pulsed pellets and isosorbide, which is used in medical preparations with non-active ingredients, cardiovascular system diseases, non-active ingredients of oil/fat/wax, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Drug-containing immediate-release pill core prescription:
[0030] Starch sucrose blank core 400g
[0031] Isosorbide Dinitrate 100g
[0032] 95% ethanol 1kg
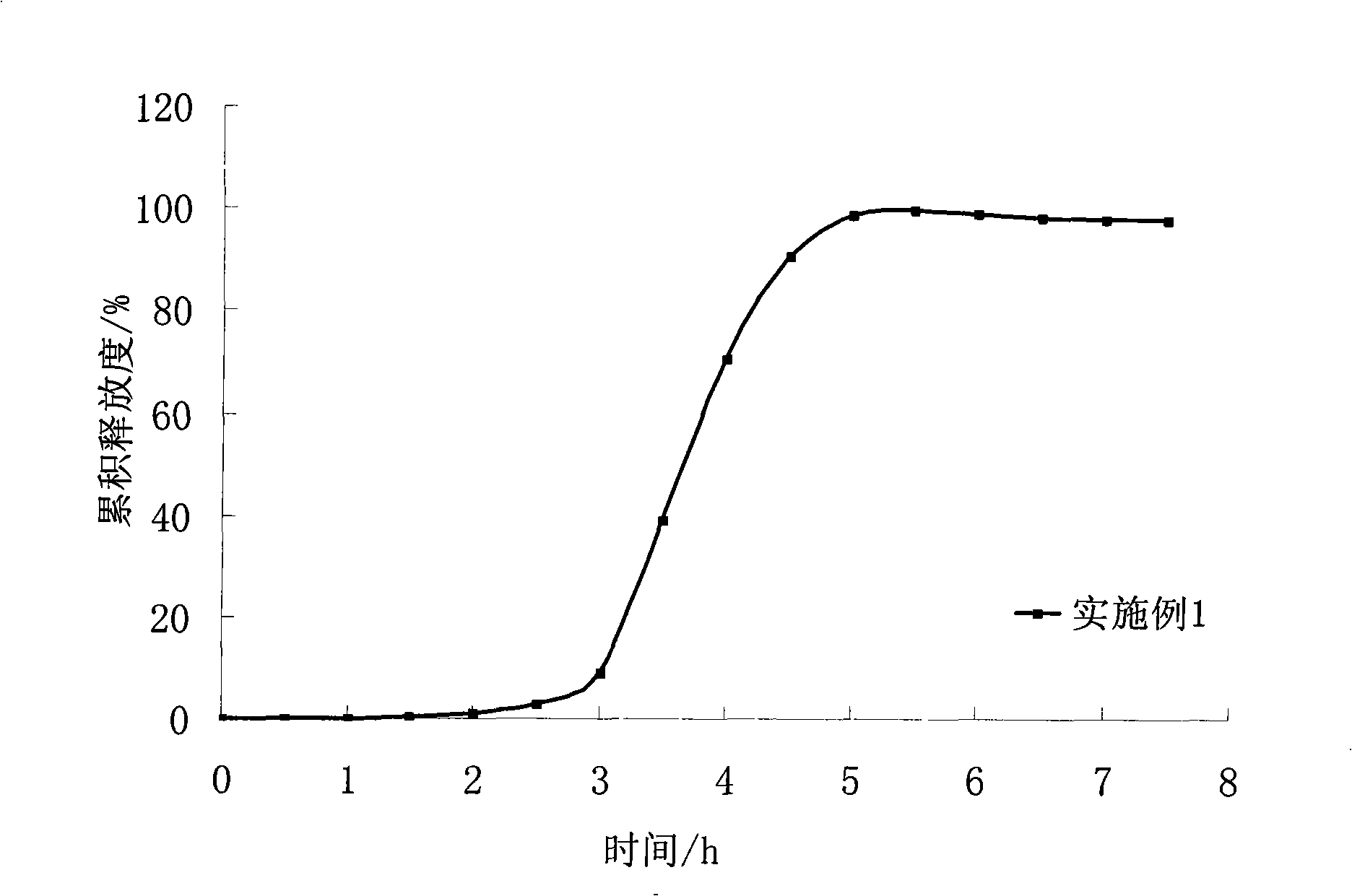
[0033] Preparation process: Dissolve isosorbide dinitrate in 95% ethanol, apply fluidized bed coating, temperature 32±1°C, flow rate 8mL / min, wrap isosorbide dinitrate on the blank core to obtain Drug immediate release pill core. The average cumulative dissolution rate was 90% in 45 minutes.
[0034] Alkaline Layer Recipe: Sodium Bicarbonate 10% (w / v), add water to 100%
[0035] Preparation process: Dissolve sodium bicarbonate in water, apply fluidized bed coating, temperature 50±1°C, flow rate 3mL / min, wrap sodium bicarbonate on drug-containing immediate-release pellet core, coating weight gain 20%, A pellet core containing an alkaline layer is prepared.
[0036] Hysteresis layer prescription w / v(%)
[0037] Polyacrylic resin III (Eudragit S100) 6.5
[0039] Add 95% ethan...
Embodiment 2
[0042] Drug-containing immediate-release pill core prescription:
[0043] Starch sucrose blank core 600g
[0044] Isosorbide Mononitrate 100g
[0045] 95% ethanol 1kg
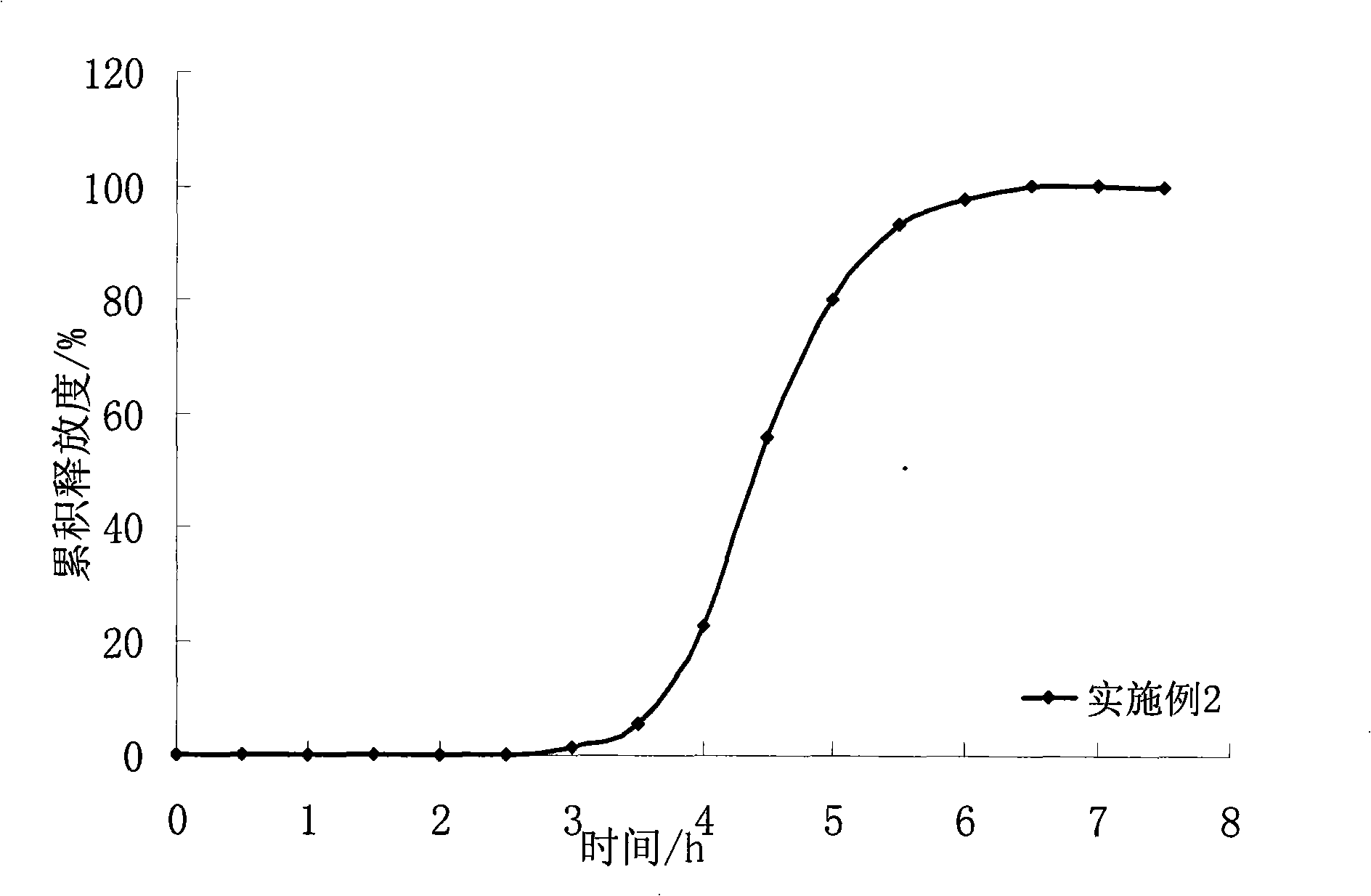
[0046] The coating process is the same as in Example 1. Get the drug-containing immediate-release pellet core. The average cumulative dissolution rate was 90% in 45 minutes.
[0047] Basic layer: Potassium dihydrogen phosphate 10% (w / v), add water to 100%, the coating process is the same as in Example 1. The weight of the coating increased by 12%, and the ball core containing the basic layer was obtained.
[0048] Hysteresis layer prescription w / v(%)
[0049] Polyacrylic resin III (Eudragit S100) 6
[0050] Micronized silica gel 1.4
[0051] Add 95% ethanol to 100%,
[0052] The coating process is the same as in Example 1. Coating weight gain of 120%. The obtained pulse pellet release curve is shown in the appendix figure 2 : Lags for about 3.5 hours, then releases completely within 2 hours.
Embodiment 3
[0054] Whole medicated immediate-release pellet core formulation w / w(%)
[0055] Isosorbide dinitrate 30
[0056] Low-substituted hydroxypropyl cellulose 40
[0057] Microcrystalline Cellulose 30
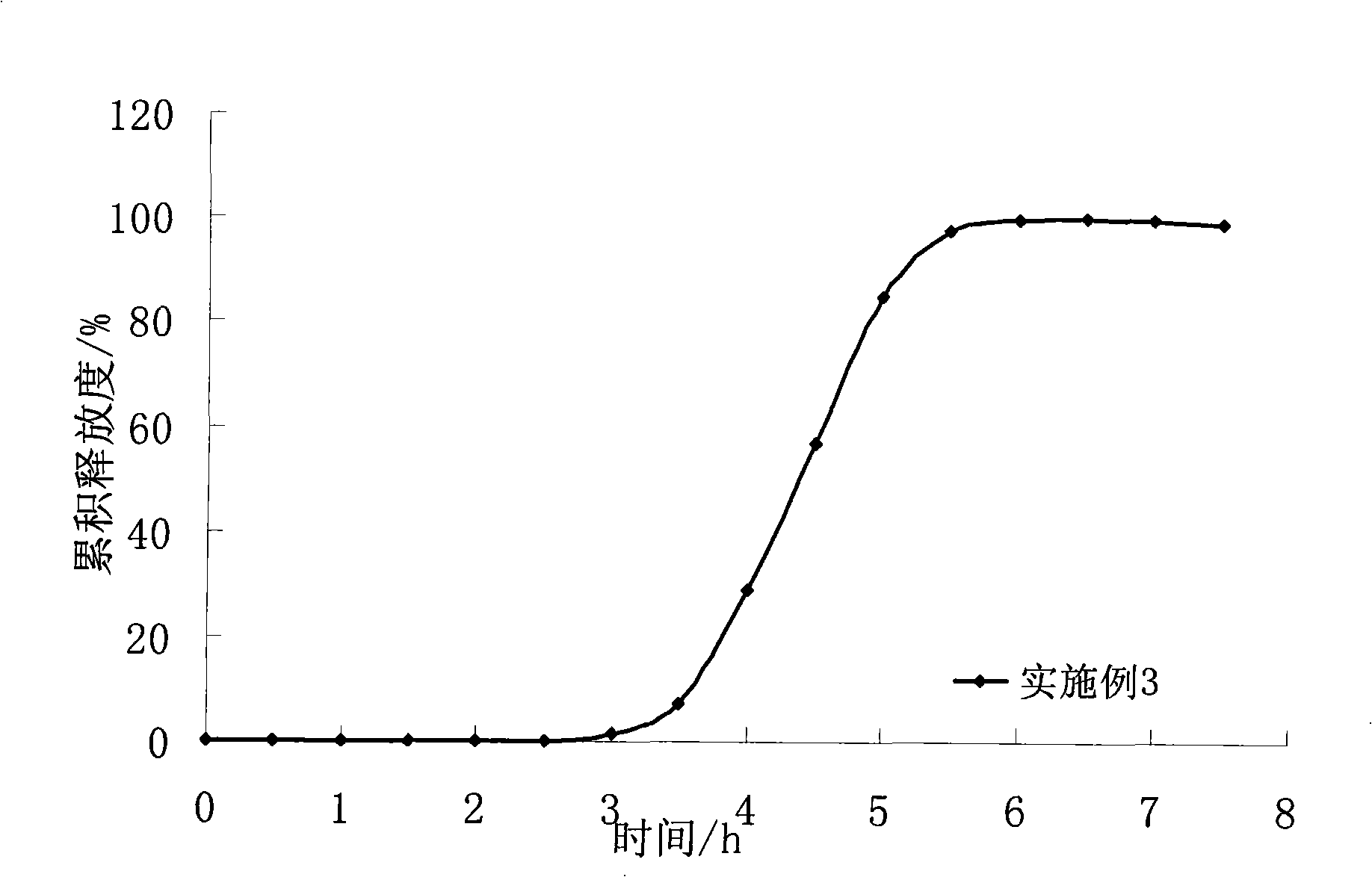
[0058] Preparation process: add 5% HPMC in 70% ethanol solution to make soft material, extrude the soft material through the sieve plate of the extruder (aperture 0.8mm), put the strip-shaped particles in the spheronizer and spheronize, and dry the pellet core at 50°C After 5 hours, sieve the 18-24 mesh drug core to obtain the drug-containing immediate-release pellet core. The average cumulative dissolution rate was 5% in 45 minutes.
[0059] Basic layer: sodium carbonate 10% (w / v), add water to 100%, the coating process is the same as in Example 1. Coating weight gain 25%;
[0060] Hysteresis layer prescription w / v(%)
[0061] Polyacrylic resin III (Eudragit S100) 8
[0062] Dibutyl sebacate 1
[0063] Glycerin 0.2
[0064] Talc 1.5
[0065] Add 95% ethanol to 100%, the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com