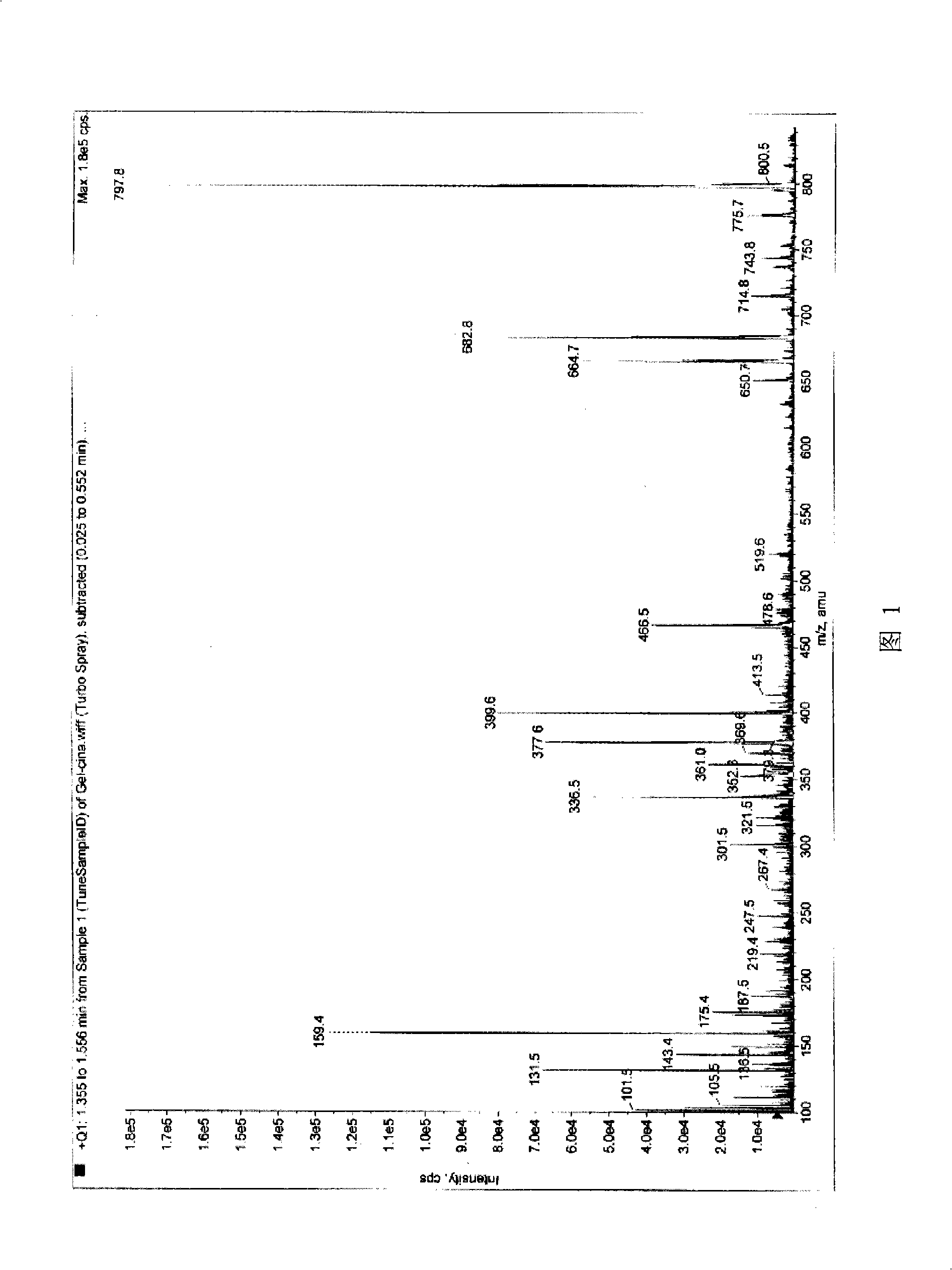
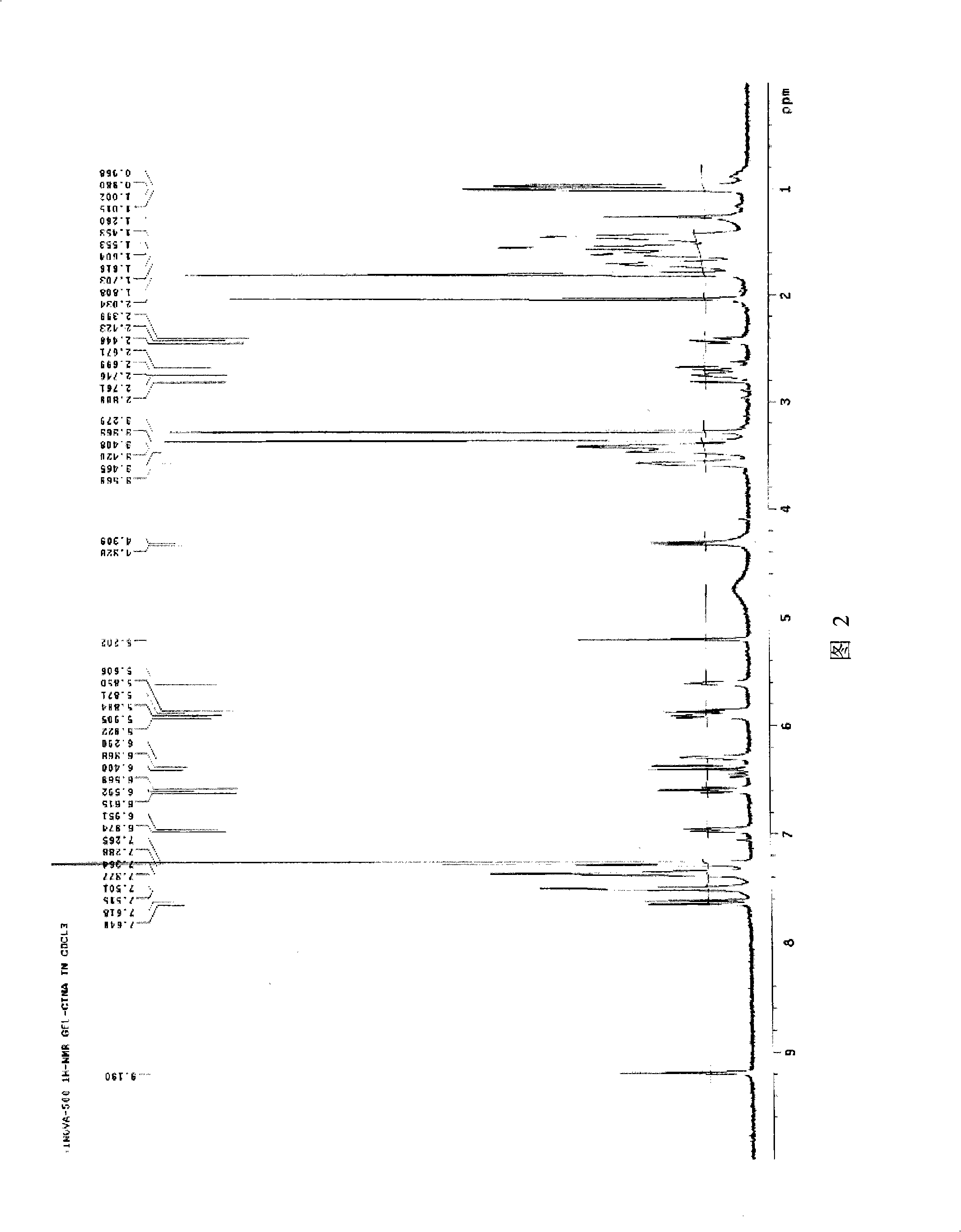
Guidemycin and use thereof in tumor treatment
A technology of guidanamycin and geldanamycin, applied in the field of geldanamycin derivatives, achieves high purity, good development value and application prospect, and the effect of inhibiting tumor cell migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] : preparation of guidamicin
[0045] Dissolve geldanamycin (Sigma product) and hexamethylenediamine in chloroform, the molar ratio of the reactants is 1:10, stir and react at room temperature for 3 hours, extract the reaction solution with water after the reaction, discard the water layer, and keep the chloroform layer . The chloroform solution was blown dry with nitrogen to obtain compound 17-hexamethylenediaminogeldanamycin (compound A).
[0046] Dissolve compound A in DMF, add cinnamic acid at a molar ratio of 1:3, and simultaneously add DIPEA (N, N-Diisopropylethylamine) and HATU (O-(7-Azabenzotriazol-1-yl)- N, N, N', N'-tetramethyluronium hexafluorophosphate), stirred and reacted at room temperature for 12-24 hours to obtain the final product (1). The final product is purified by preparative thin-layer chromatography, and the developing conditions of the developing agent are chloroform: acetone=2: 1, and R f = Purple band at 0.5. The obtained silica gel powder ...
Embodiment 2
[0048] : Determination of the purity of guidamycin
[0049] Guidemycin purity testing equipment: Agilent 1100 high performance liquid chromatography, VWD detector, chromatographic column Kromasil C-18250×4.6mm 5 μm (Dima Company), workstation operating system Chemstation.
[0050] Chromatographic conditions: mobile phase: acetonitrile-water (0.1% TFA) solution (A: water, B: acetonitrile) flow rate: 1 mL / min.
[0051] Gradient elution conditions:
[0052] Time
Solvent A(%)
Solvent B(%)
0
90
10
10
10
90
14
10
90
15
90
10
20
90
10
[0053] Detection wavelength: 254nm,
[0054] Column temperature: 28°C,
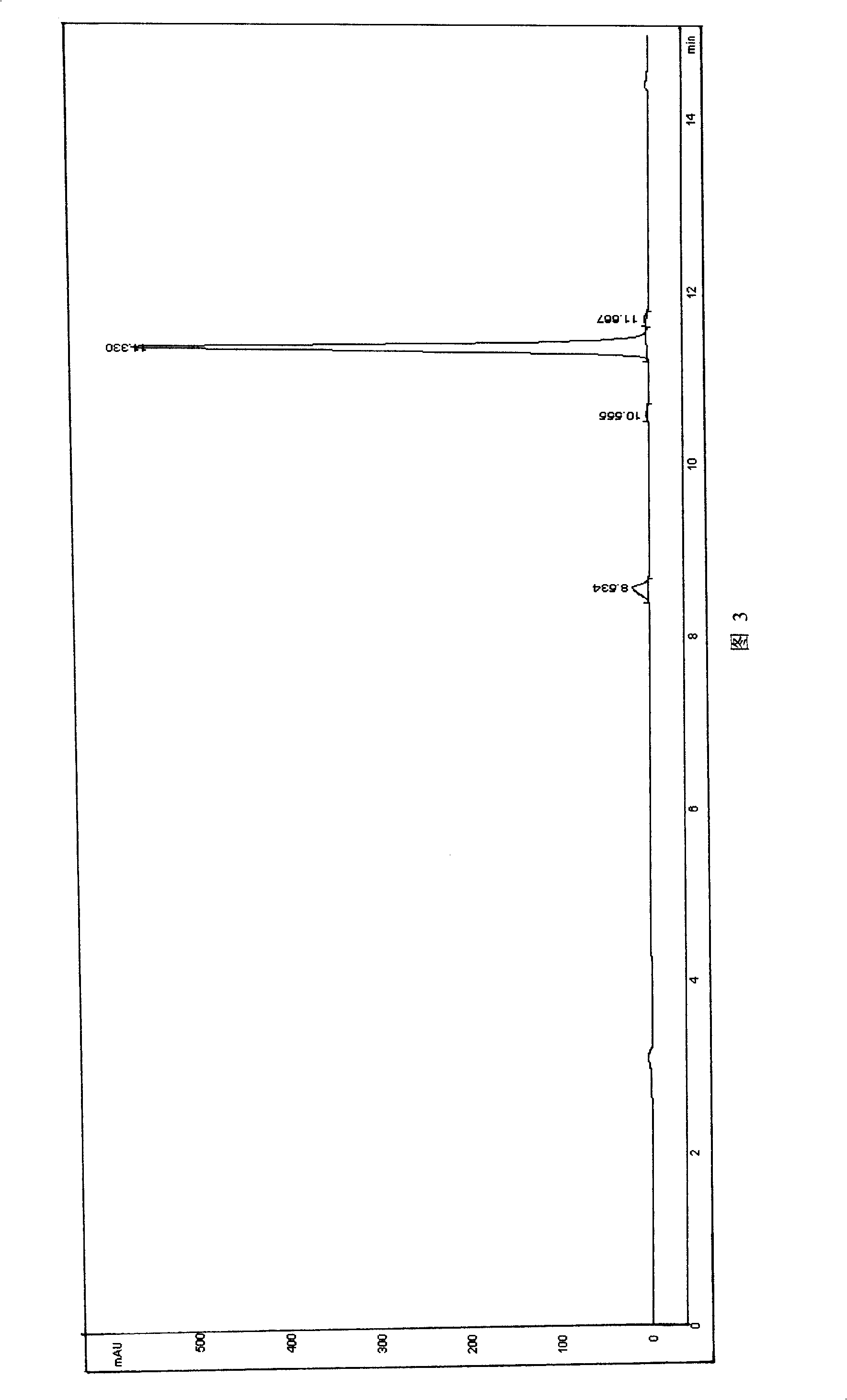
[0055] About 1 mg of guidamicin sample was dissolved in 3 ml of mobile phase to obtain guidamaycin test solution. The injection volume was 5 μl. Using the area normalization method, the purity of guidamycin was measured to be 95.06% (Fig. 3 ).
Embodiment 3
[0056] : Inhibitory effect of guidamycin on the growth of tumor cells cultured in vitro
[0057] MTT method was used to determine the inhibitory effect of guidamycin on the proliferation of human breast cancer cells MCF-7, human pancreatic cancer SW1990 cells, human liver cancer HepG2 cells, human lung cancer H460 cells, and human colorectal cancer HCT116 cells; Inhibitory effect of myelin on the proliferation of human myeloma U266 cells. The results of MTT and MTS / PMS showed that guidamicin had a significant inhibitory effect on the proliferation of tumor cells, and its IC 50 The values are listed in Table 1 respectively.
[0058] Table 1 The inhibitory effect of guidamicin on the proliferation of different tumor cells
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com