Method for producing lithium ion battery anode material vanadium lithium phosphate by sol-gel method
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve poor conductivity and cycle performance, uneven particle size distribution of synthetic materials, uneven particle size distribution of materials, etc. problems, to achieve the effect of easy control, inhibition of excessive growth, and simple and convenient methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
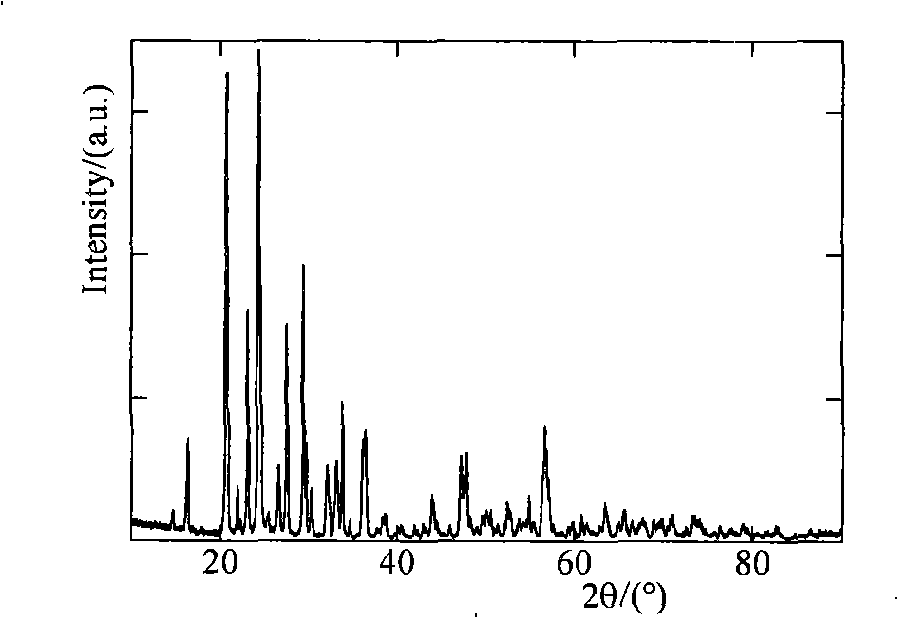
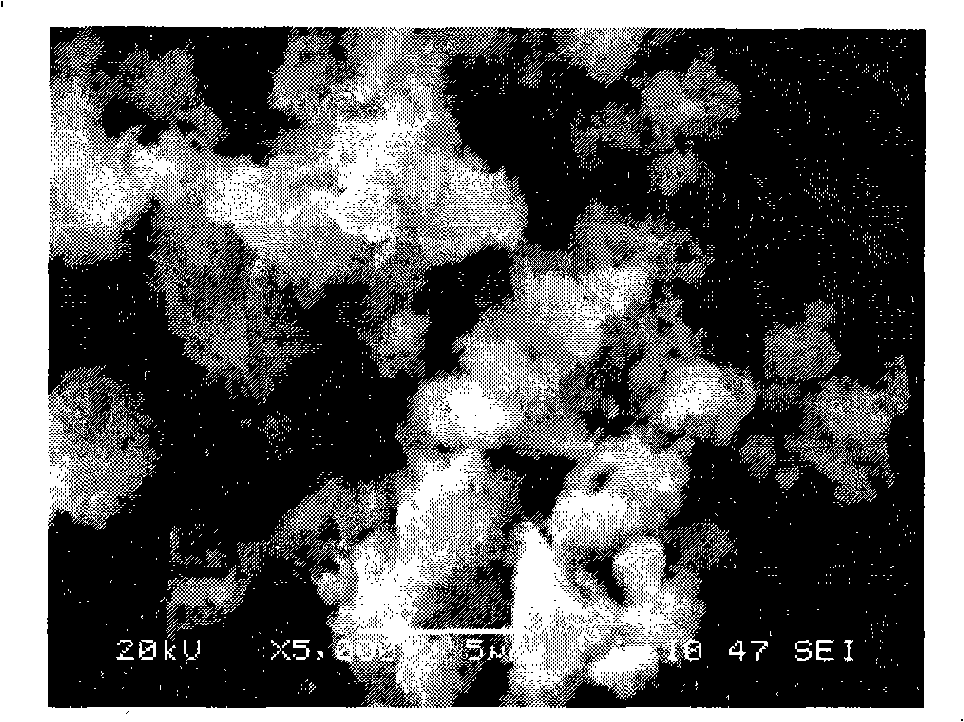
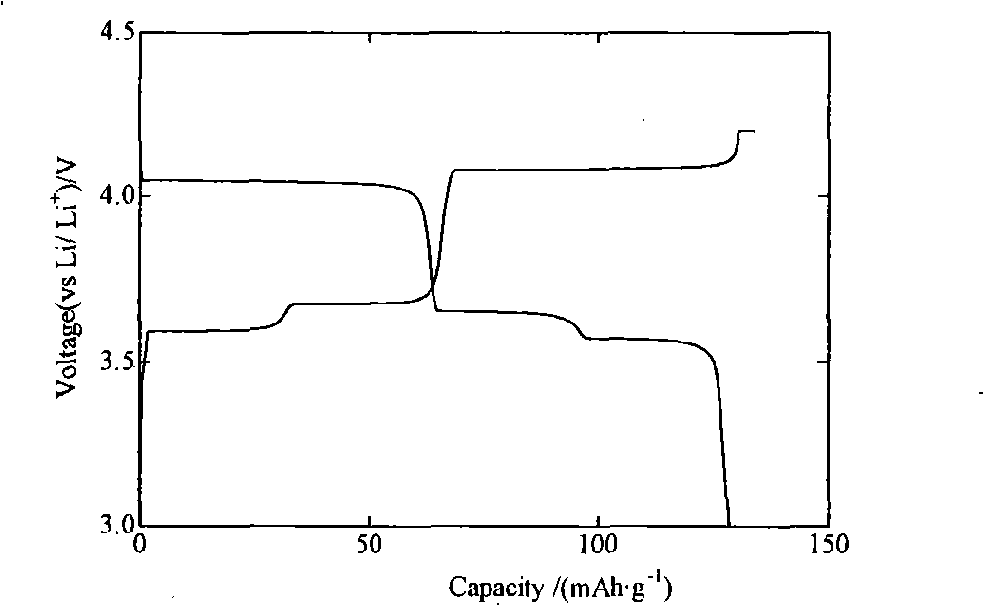
[0017] Heat 0.095mol of vanadium pentoxide powder to 600°C, keep it at constant temperature for 4 hours to make it melt, then quickly pour it into a container filled with water to form a brownish-red solution, then add 0.31mol of lithium acetate and 0.305mol of diammonium hydrogen phosphate to the solution and 0.105mol ascorbic acid, after mixing evenly, sintering at 400°C, 500°C, 600°C, and 700°C for 5 hours under the protection of nitrogen, and the finished Li 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity phase, the particle size of the product obtained by SEM is about 0.5 μm. The obtained product was assembled into an experimental button battery to measure its charge-discharge specific capacity and cycle performance. The charge-discharge was carried out at a rate of 1C. The initial discharge capacity and the discharge capacity after 30 cycles are shown in Table 1.
[0...
Embodiment 2
[0021] Heat 0.10mol of vanadium pentoxide powder to 900°C and keep the temperature constant for 1 hour to melt the vanadium pentoxide and quickly pour it into a container filled with water to form a brown-red solution, then add 0.30mol of lithium fluoride and 0.30mol of Ammonium dihydrogen phosphate and 0.10 mol of adipic acid are mixed evenly, and then sintered at 600°C under the protection of argon for 5, 10, 15 and 20 hours respectively, and the finished Li is obtained after cooling. 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity phase, the particle size of the product obtained by SEM is about 0.5 μm. The obtained product was assembled into an experimental button battery to measure its charge-discharge specific capacity and cycle performance. The charge-discharge was carried out at a rate of 1C. The initial discharge capacity and the discharge capacity after 30 cycles ar...
Embodiment 3
[0025] Heat 0.105mol of vanadium pentoxide powder to 800°C, keep it at constant temperature for 2 hours to make it melt, then quickly pour it into a container filled with water to form a brownish red solution, then add 0.305mol of lithium chloride, 0.31mol of potassium phosphate and 0.105mol of malonic acid, mixed evenly, sintered at 450°C, 550°C, 650°C, and 700°C for 20h under the protection of argon, and the finished Li 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity phase, the particle size of the product obtained by SEM is about 0.5 μm. The obtained product was assembled into an experimental button battery to measure its charge-discharge specific capacity and cycle performance. The charge-discharge was carried out at a rate of 1C. The initial discharge capacity and the discharge capacity after 30 cycles are shown in Table 3.
[0026] Experimental condition and result of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com