Three functional mutants of human phase II metabolic enzyme, construction and use thereof
A functional and metabolic enzyme technology, applied in the field of genetic engineering, can solve the problems of enhanced and weakened glucuronidation activity, complicated drug interactions, etc., to simplify the operation, save time, and improve the efficiency of recombination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
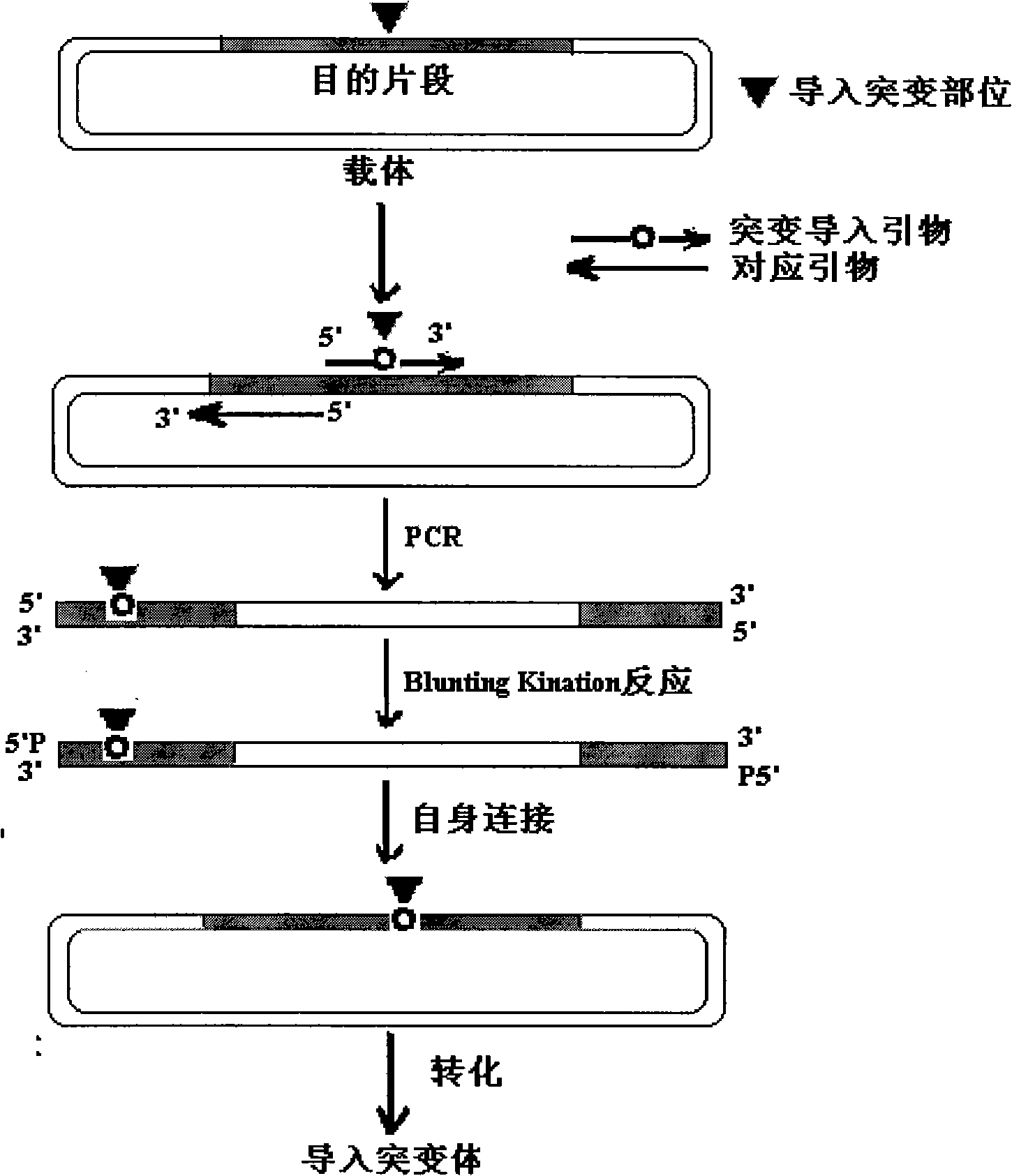
[0037] Embodiment 1 produces the plasmid of mutant recombinase: the construction of pGEM-UGT2B7*71S, pGEM-UGT2B7*3 and pGEM-UGT2B7*5
[0038] 1. Materials and methods
[0039] (1) Experimental materials
[0040] Escherichia coli strain E.coli DH5α was preserved in this experiment. PCR primers, Shanghai Sangong. The cloning plasmid pGEM-T is a Promega product.
[0041] Takara MutanBEST kit was purchased from Takara Company, restriction endonucleases, PCR product recovery kits, plasmid extraction kits, and agarose gel recovery kits were purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd. Synthesis was performed by Sangon Bioengineering Technology Service Co., Ltd., and the sequencing service was completed by Shanghai Sangon Bioengineering Technology Service Company.
[0042] (2) Experimental method
[0043] 1. Cloning construction of human UGT2B7 cDNA
[0044] Extract the mRNA of human liver tissue, take about 1 mg, add 1 ml of TRIzol reagent, and ho...
Embodiment 2
[0077] Example 2 Construction of pFastBac-UGT2B7*71S, pFastBac-UGT2B7*2 and pFastBac-UGT2B7*5
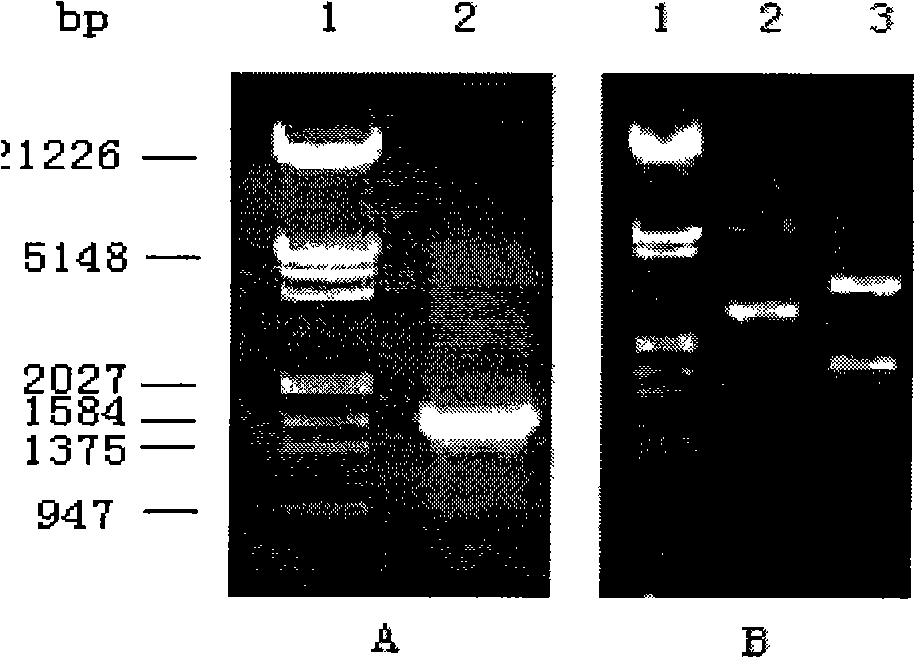
[0078] The correctly sequenced pGEM-UGT2B7*71S, pGEM-UGT2B7*2 and pGEM-UGT2B7*5 were digested with SalI / XhoI, and pFastBac TM 1-UGT2B7*1 was also digested with SalI / XhoI, the mutant target gene and vector were ligated with T4DNA ligase, transformed into E.coli DH 5α competent cells, and positive colonies were picked to extract pFastBac-UGT2B7*71S, pFastBac -UGT2B7*2 and pFastBac-UGT2B7*5 recombinant plasmids, simultaneous enzyme digestion identification, see Figure 5 . Figure 5 Middle: Lanes 1 and 10: DNA marker; Lane 2: Plasmid pFastBac-UGT2B7*1; Lane 3: Plasmid pFastBac-UGT2B7*1 digested with SalI / XhoI; Lane 4: Plasmid pFastBac-UGT2B7*71S; Lane 5: pFastBac-UGT2B7*71S plasmid was digested by SalI / XhoI; lane 6: pFastBac-UGT2B7*2 plasmid; lane 7: pFastBac-UGT2B7*2 plasmid was digested by SalI / XhoI; lane 8: pFastBac-UGT2B7*5 plasmid ; Lane 9: pFastBac-UGT2B7*5 plasmid was digeste...
Embodiment 3
[0079] Example 3 Preparation of Bacmid-UGT2B7*71S, Bacmid-UGT2B7*2 and Bacmid-UGT2B7*5
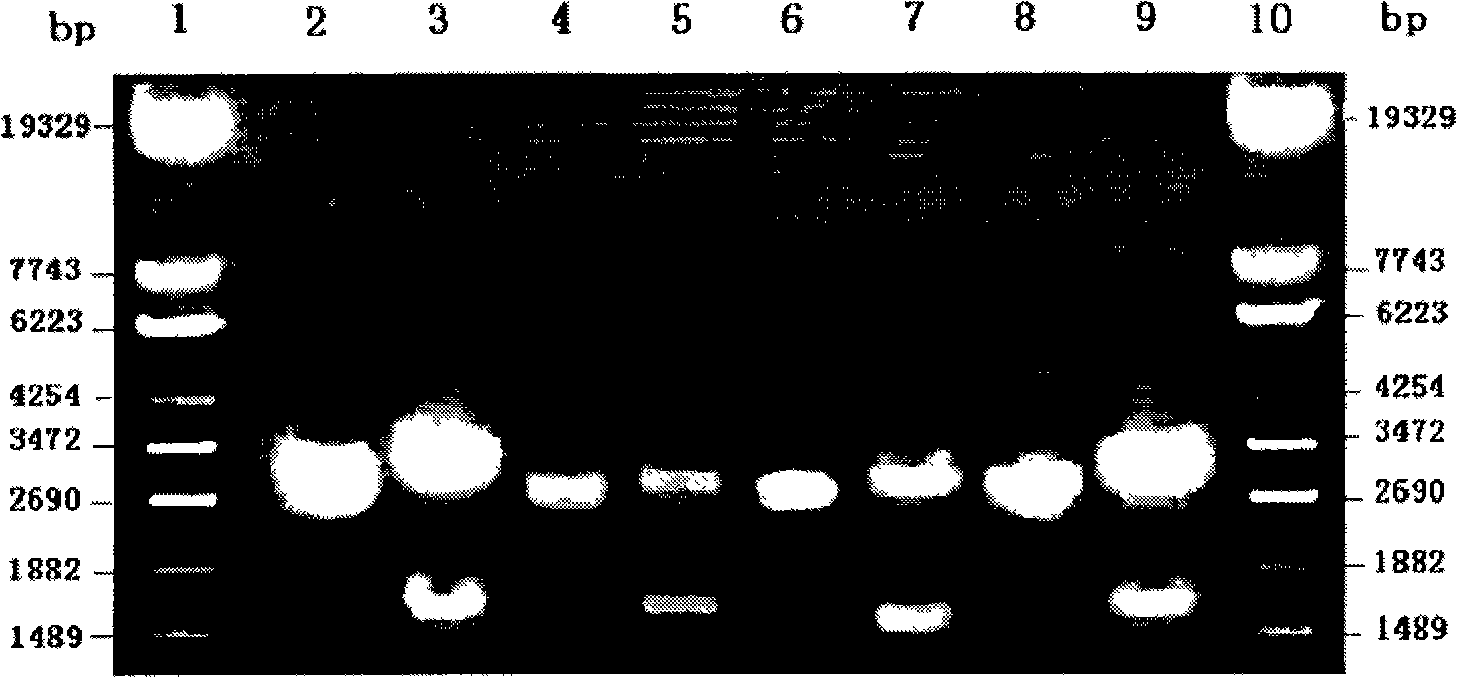
[0080] The pFastBac-UGT2B7*71S, pFastBac-UGT2B7*2 and pFastBac-UGT2B7*5 recombinant plasmids identified by restriction enzyme digestion were transformed into E.coli DH 10Bac, and the pFastBac empty plasmid was operated in parallel. After the white colonies were confirmed by blue-white screening, the transposed Bacmid-UGT2B7*71S, Bacmid-UGT2B7*2 and Bacmid-UGT2B7*5 were extracted by alkaline lysis. Extract the non-transposed Bacmid from E.coliDH 10Bac bacteria in the same way, and use M13(+) / M13(-) as primers for PCR verification. The three PCR products should have bands at about 3900, 2300, and 300bp respectively. See Image 6 , Figure 7 . Image 6 Middle: Swimming lane 1. DNA marker; Swimming lane 2. PCR result of pFastBac-UGT1A9 cosmid after transfection (3900bp); Swimming lane 3. PCR result of cosmid transfection pFastBac (2300bp); Swimming lane 4. Not transfected The PCR result of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com