Method for manufacturing spinelle lithium titanate for lithium ionic cell cathode material
A lithium-ion battery and negative electrode material technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of low initial charge and discharge efficiency, poor uniformity, and no obvious voltage indication, etc., to improve the performance of high-current charge and discharge , easy mass production, and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
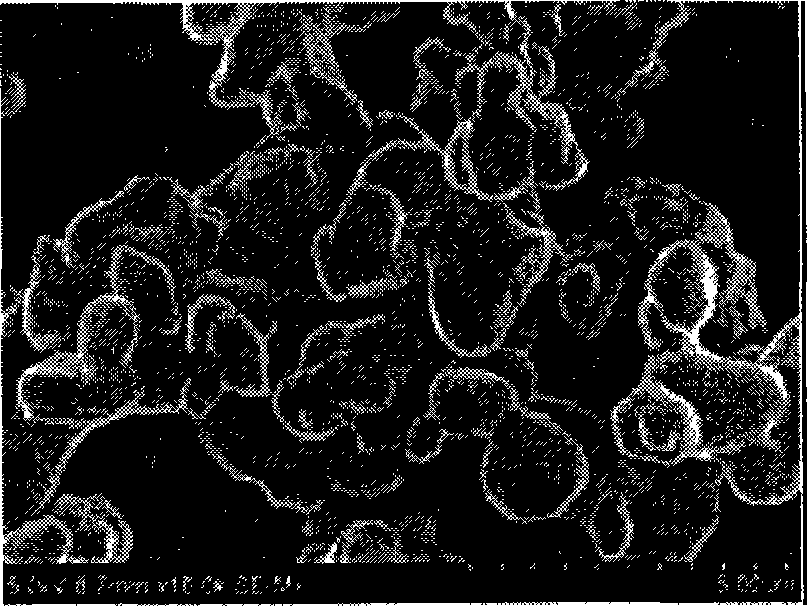
[0027] Take by weighing 93.5 g of metatitanic acid 93.5 g of purity 98% and lithium acetate (CH 3 COOLi.2H 2 (0) 84.88g was put into a mixing container, 19.82g of acetylene black was added, uniformly mixed in an ethanol medium for 10 hours, dried at 80°C, then put into an alumina crucible, treated at 300°C in a muffle furnace for 10 hours, and then Synthesized at 700°C for 15 hours to obtain lithium titanate with spinel structure. The electrochemical performance test specific capacity is 156mAh / g, and the ratio of 1C / 0.1C specific capacity is 91%. The particle morphology of the spinel structure lithium titanate that embodiment 1 obtains is as follows figure 1 As shown, the particle size of its particles is less than 500 nm. The cycle curve of the spinel structure lithium titanate that embodiment 1 obtains is as follows Image 6 shown. The rate performance of the spinel structure lithium titanate that embodiment 1 obtains is as follows Figure 7 shown, and Figure 8 Comp...
Embodiment 2
[0029] Take by weighing 93.5 g of metatitanic acid 93.5 g of purity 98% and lithium hydroxide (LiOH.H 2 O) 36.38g was put into a mixing container, 20g of polyvinyl alcohol was added, uniformly mixed in the mixture medium of ethanol and water for 20 hours, dried at 120°C, then put into an alumina crucible, and treated at 400°C in a muffle furnace for 8 hours, and then synthesized at 600°C for 25 hours to obtain spinel lithium titanate. The electrochemical performance test specific capacity is 152mAh / g, and the ratio of 1C / 0.1C specific capacity is 93%. The particle morphology of the spinel structure lithium titanate that embodiment 2 obtains is as follows figure 2 As shown, the particle size of its particles is less than 500 nm.
Embodiment 3
[0031] Weigh 93.5g of metatitanic acid with a purity of 98% and lithium carbonate with a purity of 98% and 31.05g of lithium carbonate according to the lithium-titanium molar ratio of 0.88, put them into a mixing container, add 14g of graphite, and mix uniformly in an acetone medium for 30 hours. Dry it, then put it into an alumina crucible, treat it in a muffle furnace at 500°C for 5 hours, and then synthesize it at 800°C for 5 hours to obtain spinel lithium titanate. The electrochemical performance test specific capacity is 153mAh / g, and the ratio of 1C / 0.1C specific capacity is 88%. The XRD spectrum pattern of the spinel structure lithium titanate that embodiment 3 obtains is as Figure 4 shown. The charge-discharge curve of the spinel structure lithium titanate that embodiment 3 obtains is as follows Figure 5 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com