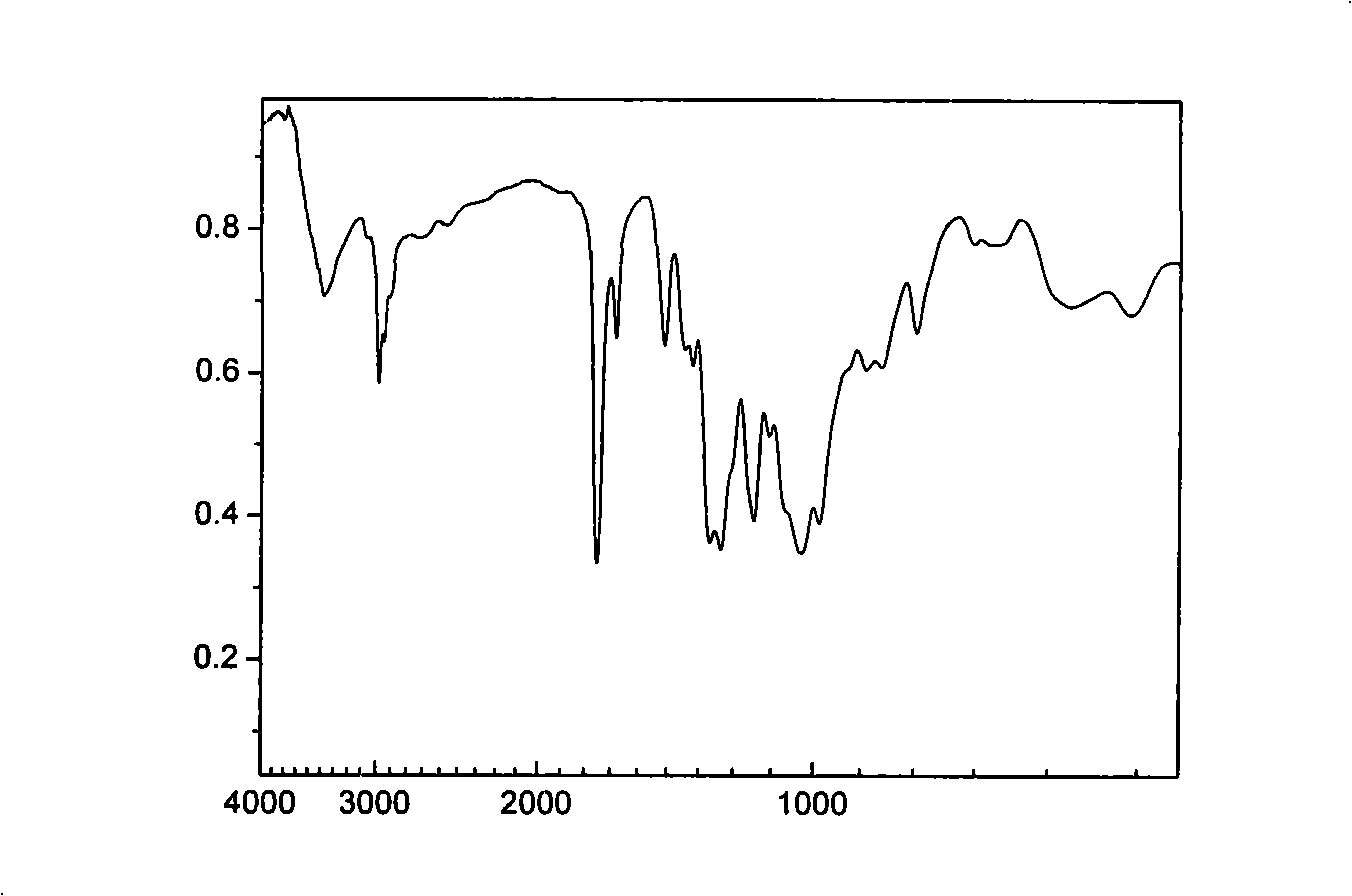
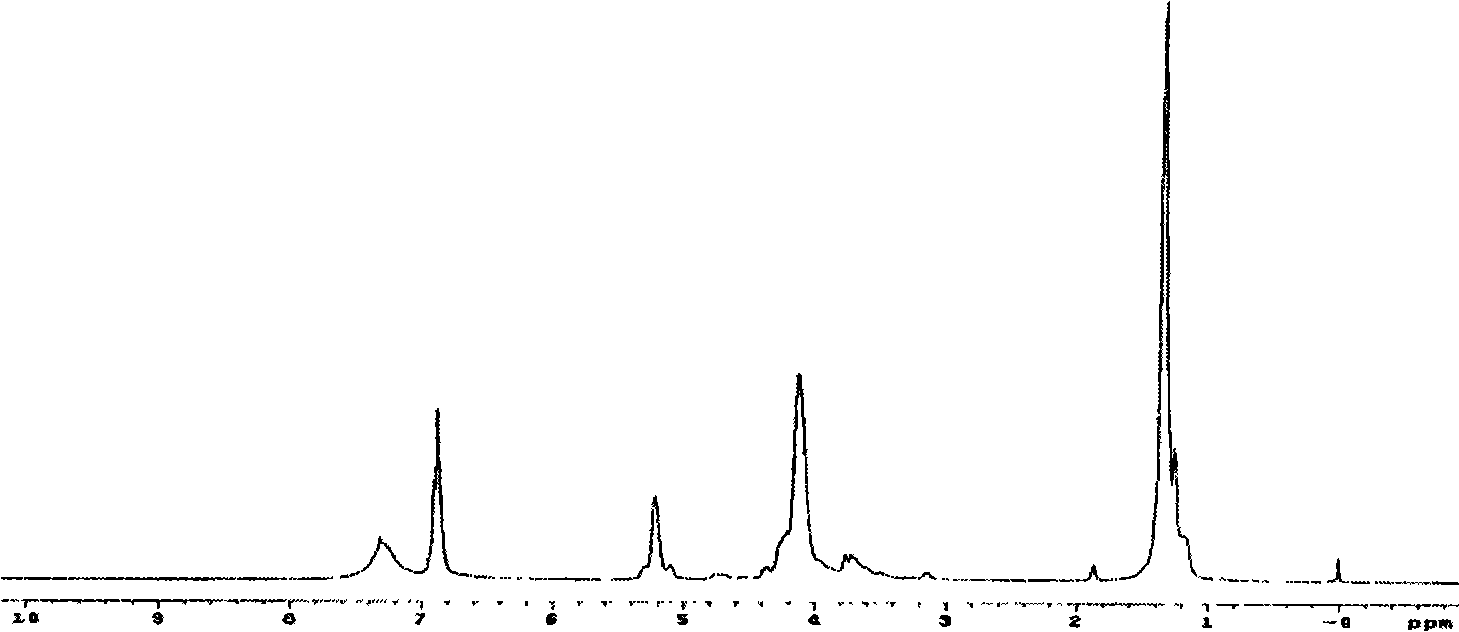
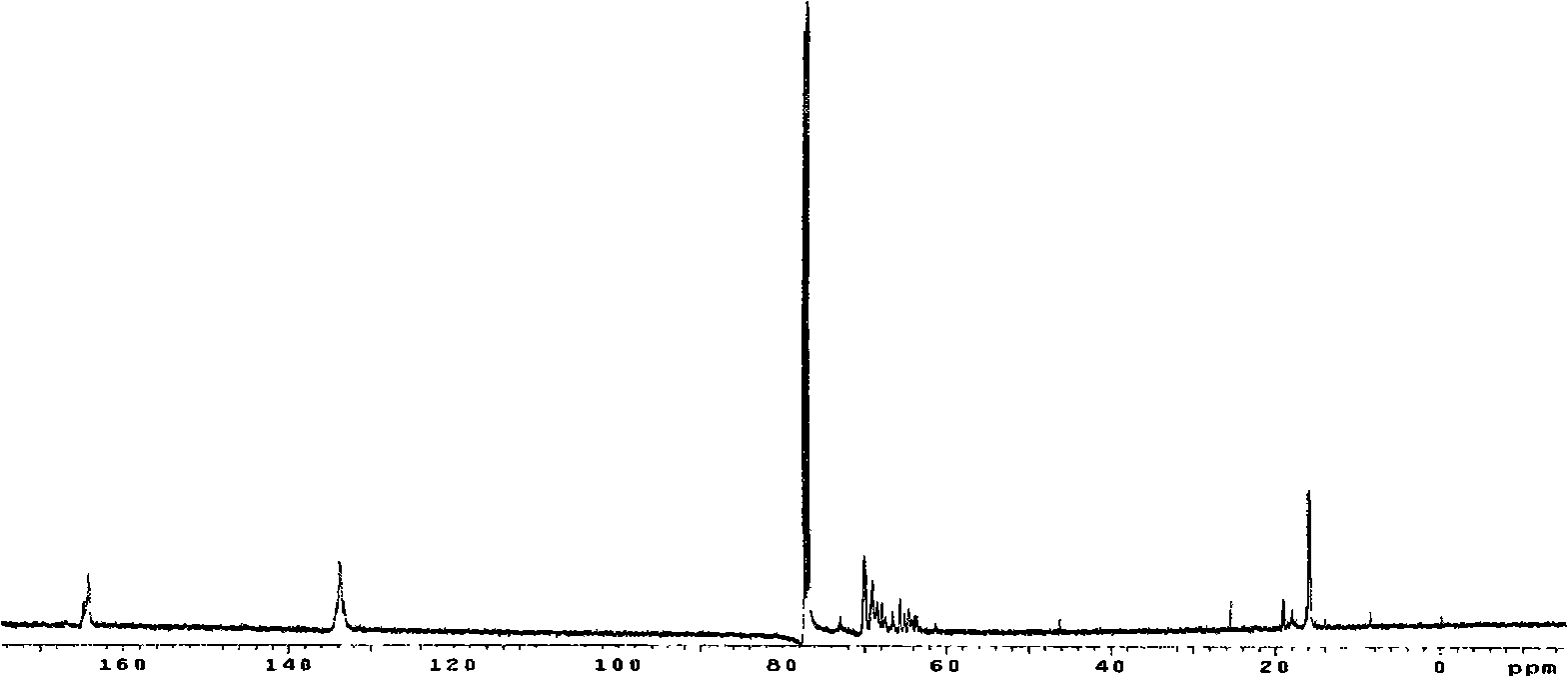
Biodegradable unsaturated polyphosphate, preparation and use method thereof
A polyphosphate and biodegradation technology, which is applied to non-active ingredients in medical preparations, drug combinations, pharmaceutical formulations, etc., can solve problems such as difficulty in filling and repairing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the synthesis of unsaturated polyphosphate-P(BPGF-EDP)
[0079] Add 116.07 grams (1.0 mol) of fumaric acid, 63.89 grams (1.1 mol) of propylene oxide and 0.79 grams (0.01 mol) of pyridine in the reaction kettle, replace the air in the kettle with nitrogen, raise the temperature to 40 ° C and stir the reaction. React under these conditions for about 8 hours, the pressure in the reactor is reduced to 0.12MPa, the temperature is raised to 90°C, and the excess propylene oxide is evaporated after continuing the reaction for 1 hour to obtain light yellow oily fumaric acid bis(1,2- propylene glycol) esters.
[0080] 92.9g (0.4mol) bis(1,2-propanediol) fumarate (BPGF) was dissolved in 1500ml dry methylene chloride, added in the reaction flask, started stirring, added 89.0g (0.88mol) of triethylamine, Nitrogen protection. The reaction bottle was placed in an ice-water bath, and under rapid stirring, 65.2 g (0.40 mol) of ethyl dichlorophosphate (EDP) dissolved in 2...
Embodiment 2
[0083] Embodiment 2: the synthesis of unsaturated polyphosphate-P(BEGF-EDP)
[0084] Add 116.07 grams (1.0 mol) of fumaric acid and 0.79 grams (0.01 mol) of pyridine into the reaction kettle, replace the air in the kettle with nitrogen, add 48.4 grams (1.1 mol) of ethylene oxide, raise the temperature to 40 ° C and stir Reaction, react under this condition for about 8 hours, the pressure in the reactor is reduced to 0.28MPa, be warmed up to 90 ℃, continue to react after 1 hour and steam the excessive oxirane, obtain light yellow oily fumaric acid bis(1 , 2-ethylene glycol) ester.
[0085] 81.0g (0.4mol) of bis(1,2-ethylene glycol) fumarate (BPGF) was dissolved in 1500ml of dry dichloromethane, added to the reaction flask, started to stir, and 89.0g (0.88mol) of triethylamine was added ), nitrogen protection. The reaction bottle was placed in an ice-water bath, and under rapid stirring, 65.2 g (0.40 mol) of ethyl dichlorophosphate (EDP) dissolved in 200 ml of dichloromethane ...
Embodiment 3
[0086] Embodiment 3: two (1,2-propanediol) fumarates - the synthesis of phenyl dichlorophosphate polymer
[0087] Except that phenyl dichlorophosphate monomer is used to replace ethyl dichlorophosphate monomer, the synthesis and separation method described in Example 1 is used to prepare bis(1,2-propanediol) fumarate-phenyl dichlorophosphate polymer .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com