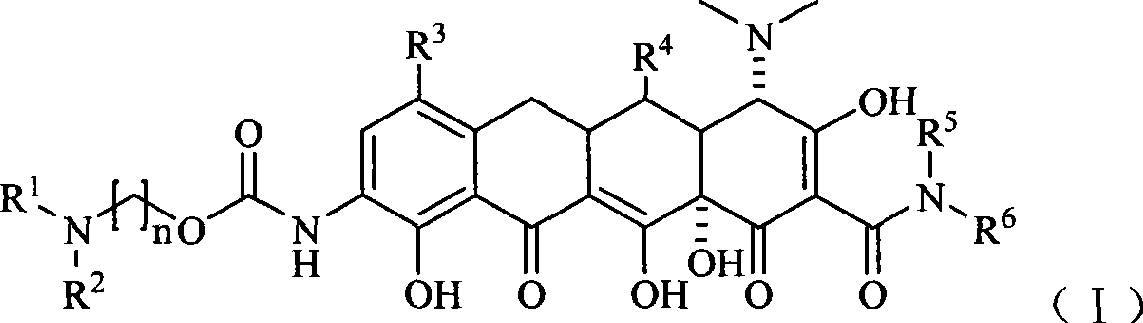
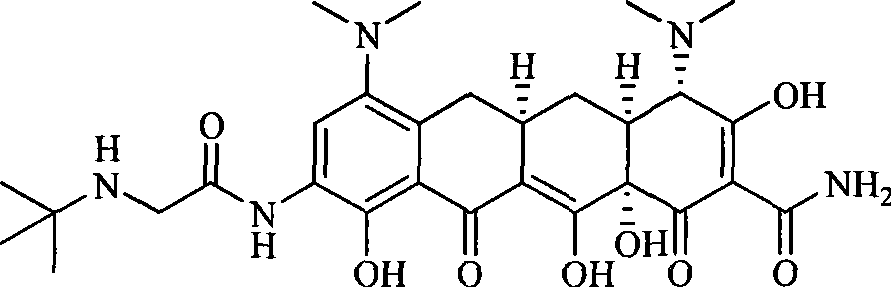
Glycinamide alkyl oxanamide tetracycline derivants
An alkyl and amino technology, applied in the field of glycine alkoxyamide tetracycline derivatives, can solve problems such as less than ideal activity of gram-negative bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 [4S-(4α, 12aα)]-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3, 10, 12, 12a- Preparation of Tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide
[0093] Throw 26.5g (50mmol) [4S-(4α, 12aα)]]-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11 in the reaction bottle, 12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthalene carboxamide dihydrochloride, dissolved in 150ml of concentrated sulfuric acid, cooled in an ice bath under stirring, Then 6.8g of sodium nitrate was added, the mixture was stirred in an ice bath for 1h, and the reaction was completed, the mixture was added dropwise to 2000ml of ether, a solid was precipitated, washed with a small amount of ether and dried, the solid was added to 100ml of ethanol, and then 2g of 10 % palladium carbon, stirred at room temperature under 2 MPa hydrogen pressure for 1.5 h, filtered, and concentrated under reduced pressure, and the residue was added with 800 ml of ether under vigorous stirring...
Embodiment 2
[0094] Example 2 [4S-(4α, 12aα)]-9-[(2-(tert-butylamino)-ethoxy)formamide]-4,7-bis(dimethylamine base)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide
[0095] At 0°C, 4.7g (10mmol) [4S-(4α, 12aα)]-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11, 12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide, a mixed solution of 6ml of dimethylacetamide and 50ml of dichloromethane, and then Add 3ml of triethylamine, stir and mix evenly, slowly add 3.2g of 2-(benzyl(tert-butyl)amino)-ethoxycarbonyl chloride / 10ml of dichloromethane solution dropwise, reflux and stir for 2h, evaporate the solvent under reduced pressure Then add 100ml of ethanol to dissolve, add 1g of 10% palladium carbon, stir under 1.5MPa hydrogen pressure at 40°C for 4h, filter, concentrate the filtrate under reduced pressure, dropwise add 100ml of ether in ice bath, precipitate solid, filter and After drying, 4.1 g of the target compound was obtained, ...
Embodiment 3
[0100] Embodiment 3 The preparation of compound freeze-dried powder injection of the present invention
[0101] 1. Prescription:
[0102]
[0103] 2. Preparation process: clean, sterilize, and depyrogenate the vials, rubber stoppers, liquid-dosing containers, instruments and equipment used in production; weigh the raw material compound 1, vitamin C, and mannitol according to the prescription, add water, stir and dissolve , an appropriate amount of hydrochloric acid to adjust the pH, and then add activated carbon for needles with a dosing volume of 0.05%, stir for 15 minutes, filter, and decarbonize; add water for injection to the full amount, and constant volume; Clarity; inspection of semi-finished products; dispensing 2ml of liquid medicine into vials, half-tamped; put the sample in a freeze dryer, and freeze-dry using the following freeze-drying process: -40°C pre-freeze for 4 hours, -40~0°C Low-temperature vacuum drying for 18 hours, heating and drying at 0-30°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com