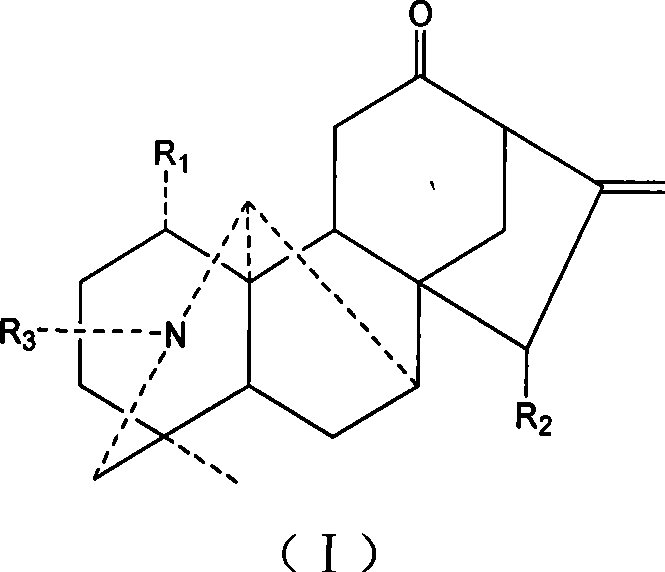
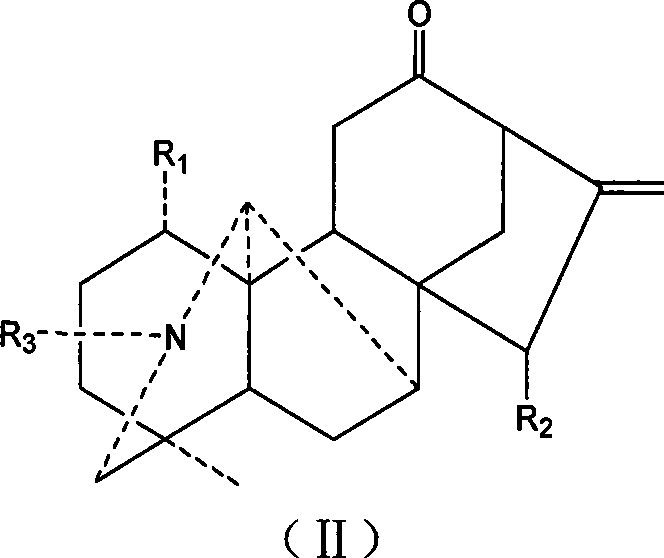
Aconite napelline type diterpene alkaloid acylate, synthesis and use thereof
A technology of alkaloid acylate and diterpene, applied in the direction of chemicals, applications, and biocides for biological control, which can solve the problems of unreported insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Dissolve 46.6mg of songuoling in 1ml of pyridine, then add 0.5ml of acetic anhydride dropwise, and stir at room temperature for 20 hours. Adjust the pH to 9 with saturated sodium carbonate solution. After extraction with chloroform, the chloroform layer was washed three times with water and saturated brine, dried, and distilled under reduced pressure to obtain 53 mg of 1-0-acetyl-15-0-acetyl-songuoling.
[0010] The product structure was confirmed by mass spectrometry, EI-Ms m / z: 442 (M + );
[0011] NMR testing, 1 HNMR (CDCl 3 ) (ppm): 5.64, 5.25 (each1H, s, extracyclic double-bonded hydrogen), 2.11, 2.03 (each3H, s, COCH 3 ), 3.15 (3H, t, NCH 2 CH 3 ), 0.75 (3H, s, 18-CH 3 );
[0012] NMR testing, 13 CNMR (CDCl 3 )δ: 208.8, 170.6, 170.3, 145.2, 112.9, 50.6, 13.5 (NCH 2 CH 3 ).
Embodiment 2
[0014] Dissolve 37.9 mg of songuoling in 1 ml of pyridine, then add 0.5 ml of propionic anhydride dropwise, and stir at room temperature for 20 hours. The saturated sodium carbonate solution was used to adjust the pH to 9. After extraction with chloroform, the chloroform layer was washed three times with water and saturated saline, dried, and distilled under reduced pressure to obtain 38.1 mg of a propionylation mixture of sorgolin. Put on a silica gel column and elute with petroleum ether / acetone=5:1 to obtain 28.3 mg of 1-0-propionyl-15-0-propionyl sorgoline.
[0015] The product structure was confirmed by mass spectrometry, EI-Ms m / z: 470.3 (M + );
[0016] NMR testing, 1 HNMR (CDCl 3 )(ppm): 5.66(1H, s), 5.25(1H, d), 5.03(1H, dd), 4.99(1H, s), 2.75(1H, q), 2.58(1H, m), 2.53(1H , d), 2.44(1H, m), 2.30(3H, m), 2.21(1H, d), 2.18(1H, m), 2.13(3H, s), 2.02(1H, m), 1.94(1H, m), 1.60(4H,m), 1.43(1H,dd), 1.41(1H,d), 1.23(3H,t), 1.19(3H,t), 1.15(3H,t)
Embodiment 3
[0018] Determination of insecticidal activity: Weigh a certain mass of the original drug with an analytical balance (0.0001 g), add a solvent (DMF) containing 0.1% Tween-80, and prepare a 5% preparation. Take a certain amount of preparation, add distilled water (acetone for spot method dilution) to dilute and prepare the medicinal solution for measuring the required concentration, and the general sieve concentration is 500mg / L. Cut off both ends of broad bean leaves, put the back side up on a small piece of cotton, put it in a plastic petri dish, add a small amount of water, and pick up alfalfa aphids to produce nymphs. After 24 hours, the adult aphids were removed, and after continuing to cultivate for 2 days, the leaves were fully soaked in the medicinal solution for 5 seconds, then placed on cotton again, and dried naturally. Carbosulfan (10 mg / L) and blank CK were used as controls. After 24 hours, the results showed that 1-0-acetyl-15-0-acetyl sorgoline and 1-0-propionyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com