Polythioether acid imide and preparation thereof
A technology of polythioetherimide and phthalimide, which is applied in the field of preparing new polythioetherimide, can solve the problems of cumbersome preparation process and high polymer cost, and achieve reasonable and practical process and low cost , the effect of low melt viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
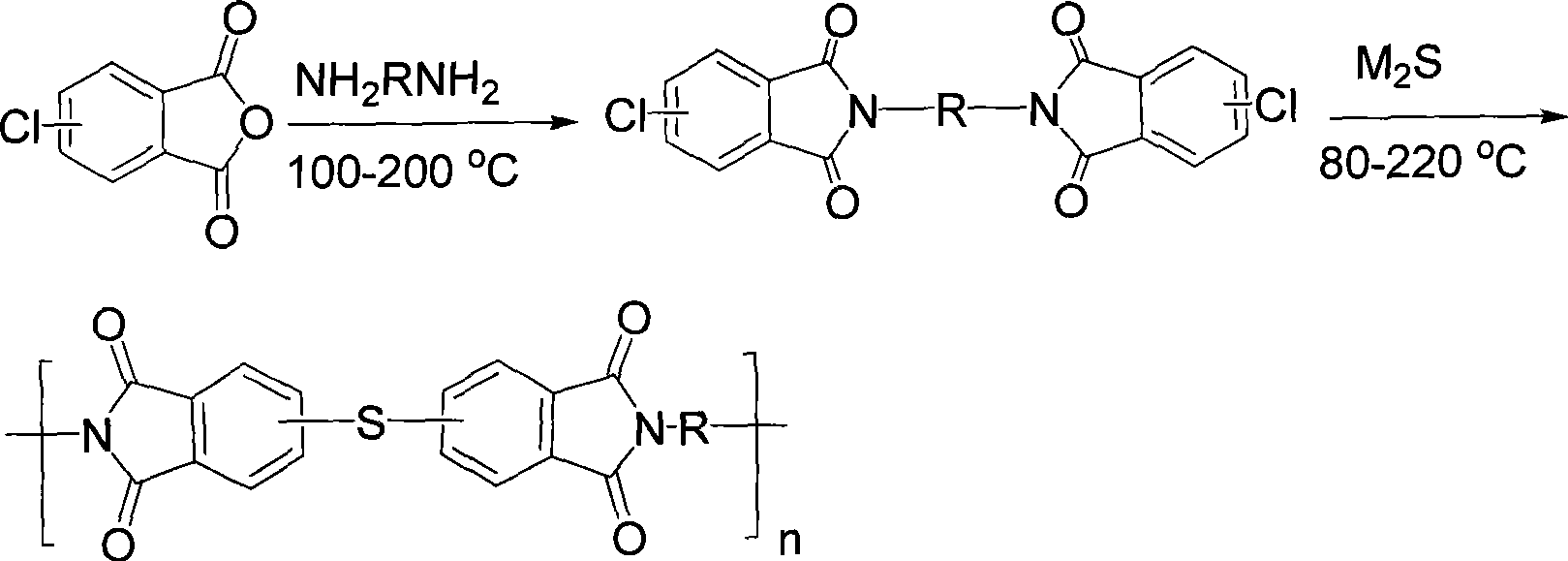
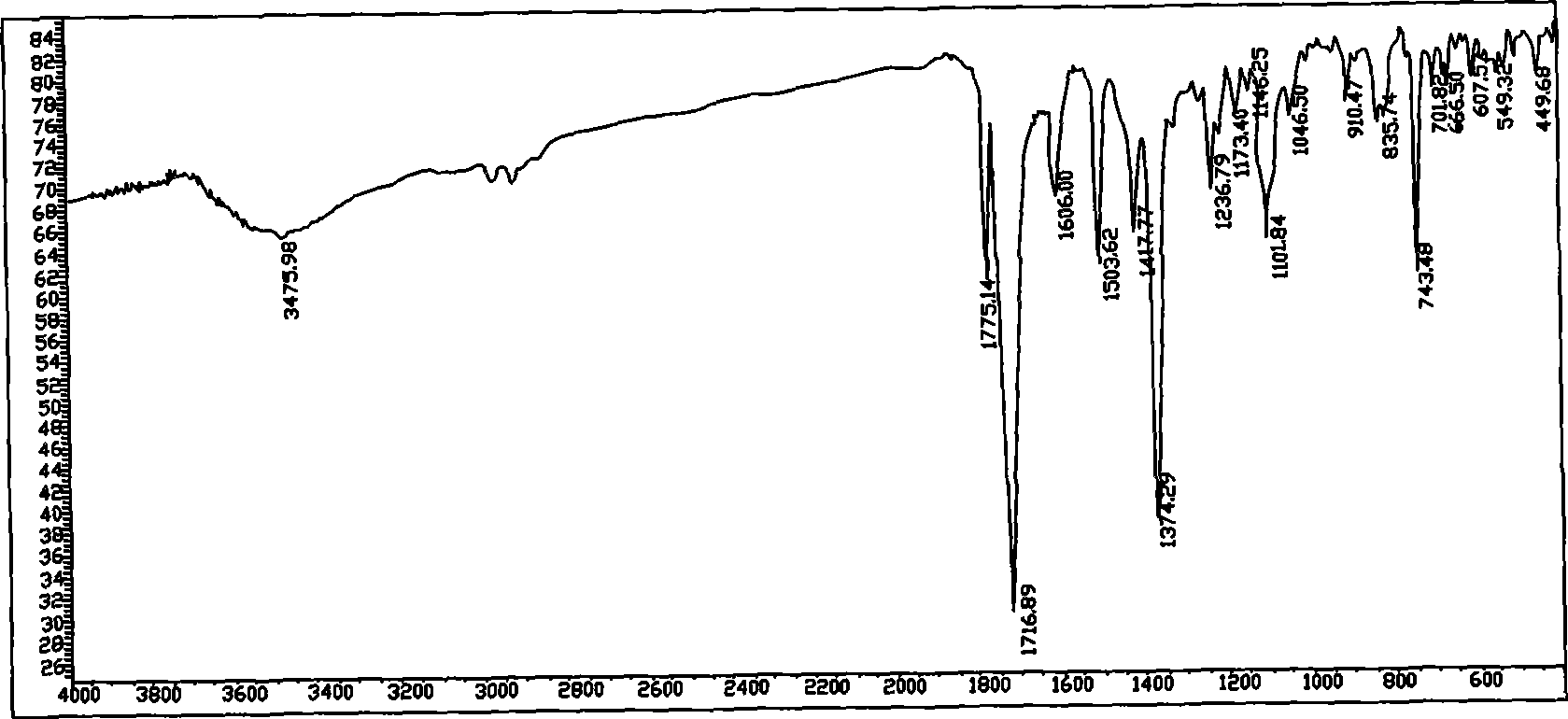
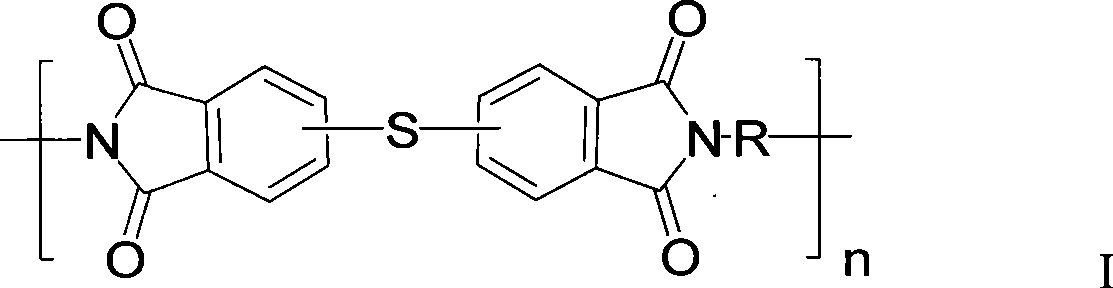
[0032]Add 182.56 grams (1.0mol) of 4-chlorophthalic anhydride and 1000 milliliters of glacial acetic acid into a dry and clean 2L three-necked flask, stir to dissolve and add 113.16 grams (0.5mol) of 3,3'-dimethyl-4,4'- Diaminodiphenylmethane (DMMDA), heated to 140°C for 24 hours, then cooled to room temperature, poured it into 10 liters of water, filtered to obtain a white solid, washed the filter cake three times with distilled water, and dried under vacuum at 120°C to obtain double Chlorine monomer crude product 249.9 grams, yield 90%. The crude product was recrystallized from dimethyl sulfoxide and used in the next polymerization reaction. Under an argon atmosphere, add 27.77 grams (0.05mol) of the above-mentioned dichloro monomer, 3.90 grams (0.05mol) of anhydrous sodium sulfide, 2.000 grams (0.05mol) of sodium hydroxide, and 300 milliliters of N , N'-dimethylacetamide (DMAc), heated to 120 ° C for 24 hours, cooled to room temperature, slowly poured the reaction solution...
Embodiment 2
[0034] Add 182.56 grams (1.0mol) of a mixture of 3-chlorophthalic anhydride and 4-chlorophthalic anhydride with a mass ratio of 5:1, 1000 milliliters of glacial acetic acid, and stir to dissolve and add 113.16 grams (0.5mol ) DMMDA, heated to 130 ° C for 24 hours, then cooled to room temperature and poured it into 10 liters of water, filtered to obtain a white solid, the filter cake was washed three times with distilled water, and vacuum-dried at 120 ° C to obtain 236 grams of dichloromonomer crude product , yield 85%. The crude product was recrystallized from a mixed solvent of DMAc and toluene (volume ratio 2:1) and used for the next polymerization reaction. Under a nitrogen atmosphere, add 27.77 grams (0.05mol) of the above-mentioned dichloromonomers, 3.90 grams (0.05mol) of anhydrous sodium sulfide, 6.36 grams (0.06mol) of anhydrous sodium carbonate in a dry and clean 500 milliliter three-necked flask, and 250 milliliters DMAc, heated up to 130°C for 36 hours, cooled to r...
Embodiment 3
[0036] Add 91.28 grams (0.5 mol) of a mixture of 3-chlorophthalic anhydride and 4-chlorophthalic anhydride in a dry and clean 1L three-necked flask with a mass ratio of 3:1, 500 ml of DMAc, stir to dissolve and add 49.56 grams (0.25mol) 4,4'-diaminodiphenylmethane (MDA), heated to 80°C for 2 hours, then heated to 130°C for 16 hours, then concentrated under reduced pressure to about 200 ml, poured into 3 liters of water, filtered to obtain White solid, the filter cake was washed three times with distilled water, and vacuum-dried at 120° C. to obtain 116 grams of crude dichloromonomer, with a yield of 88%. The crude product was vacuum melted and used for the next step of polymerization. Under a nitrogen atmosphere, add 26.37 grams (0.05mol) of the above-mentioned dichloromonomer, 2.30 grams (0.05mol) of anhydrous lithium sulfide, and 200 milliliters of N-methyl-2-pyrrolidone (NMP) in a dry and clean 500 milliliter three-necked flask. , heated up to 170°C for 8 hours, cooled to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inherent viscosity | aaaaa | aaaaa |
| Inherent viscosity | aaaaa | aaaaa |
| Inherent viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com