Catalyst for H2 selective oxidation in styrene production
An oxidation reaction and catalyst technology, which is applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, etc., can solve the problems of short service life and high loss rate of raw material aromatics, and achieve Effect of high combustion activity, shortened residence time, and short diffusion paths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
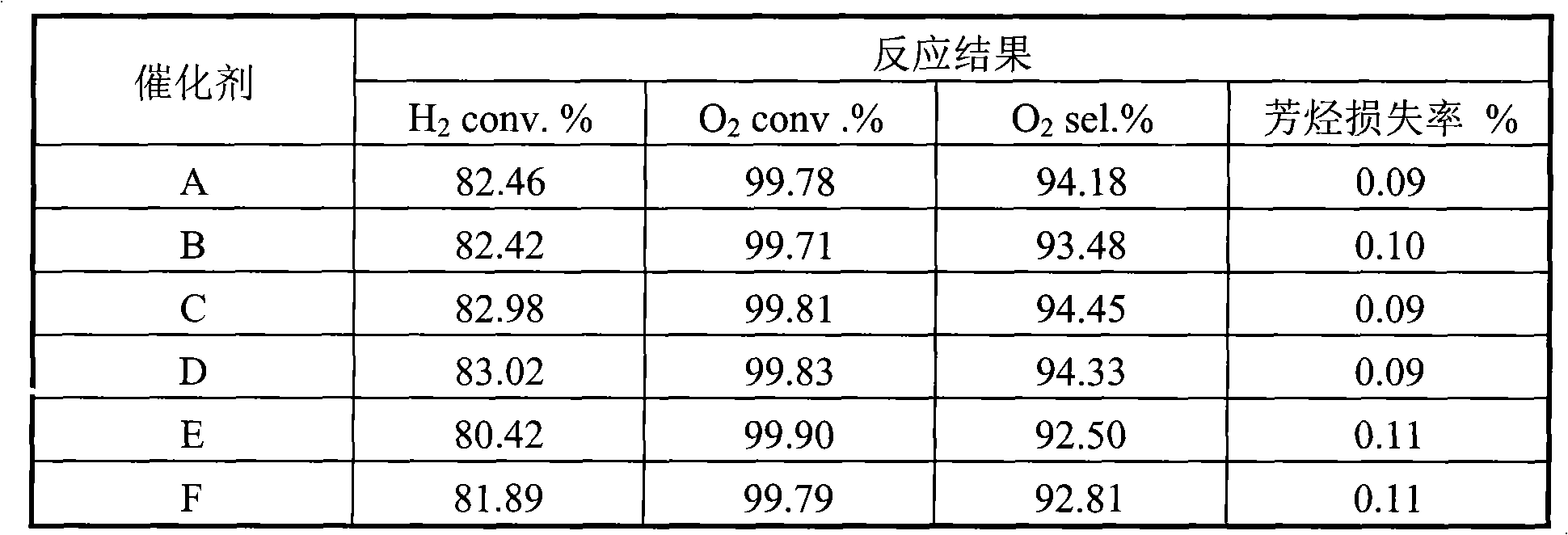
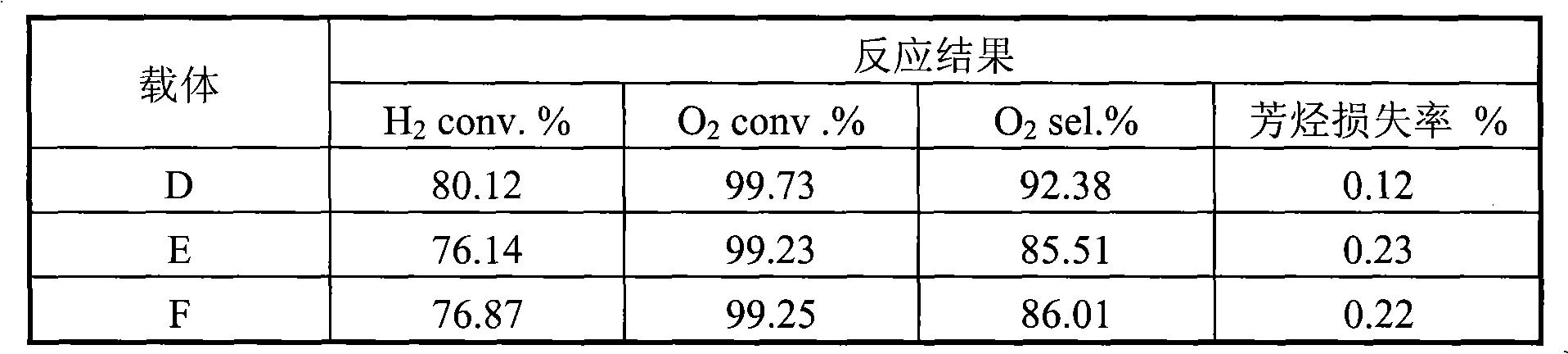
Embodiment 1
[0016] 40 grams of alumina sol (containing 15% alumina by mass ratio), 60 grams of 2% glycerin solution, and 0.5 grams of Span 80 were made into a slurry. Then add 0.5 grams of potassium feldspar and 40 grams of γ-Al with a particle size of 100 microns or less to this mixture. 2 o 3 powder (specific surface area 200 m 2 / gram). After stirring for about ten minutes, add 0.85 g of Ce 2 o 3 , 2.2 grams of barium oxide and 0.2 grams of germanium dioxide, and the resulting slurry was ball milled at room temperature for 4 hours so that the particle size was controlled below 10 microns. Slurry spraying to MgAl particle size 4mm 2 o 4On the pellets, dry at 80°C for 2 hours, then heat up to 100°C and dry again for 2 hours, and finally bake at 1100°C for 6 hours to obtain a layered composite carrier. Scanning electron microscopy shows that the thickness of the coating is about 90 microns, and the specific surface area of the coating is 165 meters 2 / gram.
[0017] Dissolve ch...
Embodiment 2
[0019] SnCl 2 and praseodymium chloride are dissolved in water according to the molar ratio of 1:0.5, and the above solution is impregnated in θ-Al 2 o 3 (solid-to-liquid ratio 1:2) on the powder, dried at 150°C for 2 hours, and calcined at 400°C for 4 hours. 40 grams of alumina sol (containing 15% alumina), 60 grams of 3% polyacrylamide solution, and 0.4 grams of betaine were made into slurry. Then add 0.3 grams of calcium silicate, 40 grams of θ-Al impregnated with Sn and Pr with a particle size of less than 100 microns to this mixture. 2 o 3 powder. After stirring for about ten minutes, add 2.0 g of 25% MgCl 2 As an aqueous solution, the resulting slurry was ball milled for 4 hours at room temperature to control the particle size below 20 microns. Slurry spraying to α-Al with a particle size of 4 mm 2 o 3 On the pellets, dry at 80°C for 2 hours, then heat up to 150°C and dry again for 2 hours, and finally bake at 800°C for 10 hours to obtain a layered composite carr...
Embodiment 3
[0022] 35 grams of alumina sol (containing 25% mass ratio of alumina), 5 grams of 40% silica sol, 60 grams of 4% cyclodextrin solution, 2.0 grams of lanthanum oxide, 1.0 grams of hexadecyl trimethyl bromide ammonium chloride to make a slurry. Then add 0.4 grams of calcium silicate, 0.3 grams of potassium carbonate, 0.4 grams of lead oxide and 40 grams of δ-Al with a particle size of 100 microns or less to this mixture. 2 o 3 pink. After stirring for about ten minutes, 2.0 g of 10% neodymium chloride aqueous solution was added, and the obtained slurry was ball milled at room temperature for 4 hours to control the particle size below 10 microns. The slurry was sprayed onto mullite spheres with a particle size of 4 mm, dried at 80°C for 2 hours, then heated to 150°C for another 2 hours, and finally calcined at 900°C for 6 hours to obtain a layered composite carrier. The scanning electron microscope shows that the thickness of the coating is about 100 microns, and the specific ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com