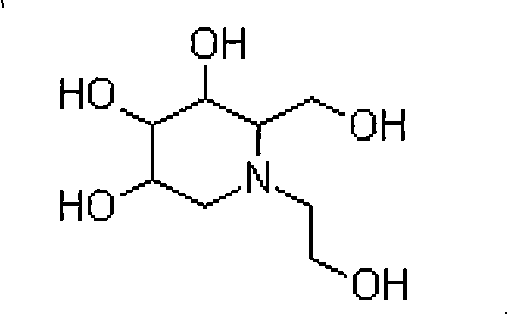
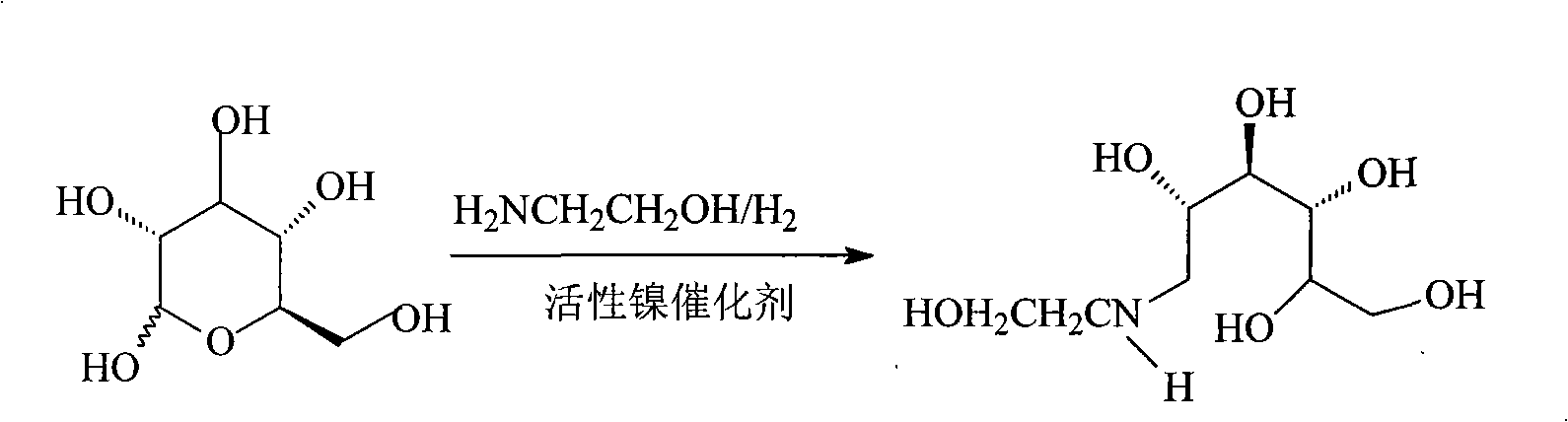
Process for producing miglitol key intermediate
A technology of refining method and treatment method, which is applied to the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxyl compounds, etc., can solve the problems of low reaction conditions, reduced catalyst activity, unfavorable hydrogenation reaction, etc. Conducive to industrial production and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 2100ml of methanol (5% water content), 1500g of glucose, 512g of ethanolamine and 150mg of triethylamine into a 10L autoclave, replace with nitrogen and add 150g of Raney-Ni (W-6 type), tighten the feeding port, and feed high-purity hydrogen, Keep the pressure at 3-3.5MPa, slowly raise the temperature to 45°C, stir the reaction for hydrogenation, until the hydrogen absorption of the reaction is not obvious (the pressure gauge needle of the autoclave will no longer drop), about 6 hours, the reaction is completed, filter while hot to remove The catalyst was cooled to room temperature and then added with 1400 ml of ethyl acetate. After crystallization for 4 hours, suction filtration and drying were performed to obtain the product N-hydroxyethylglucamine.
[0028] The crude N-hydroxyethyl glucosamine was heated with ethyl acetate: methanol = 1: 2 (volume ratio), 5% activated carbon carbon by weight of the crude product was added, stirred and refluxed for 1 hour, the acti...
Embodiment 2
[0031] Add 2250ml of anhydrous methanol, 1500g of glucose, 508g of ethanolamine and 150mg of DMF to the 10L high pressure reaction kettle, replace with nitrogen and then add 150g of Raney-Ni (T-1 type), tighten the feeding port, feed high-purity hydrogen, and keep the pressure at 3-4.2 MPa, slowly raise the temperature to 50°C, stir the reaction for hydrogenation, until the hydrogen absorption of the reaction is not obvious (the pressure gauge needle of the autoclave will no longer drop), about 8 hours, the reaction is completed, filter while hot to remove the catalyst, and cool to room temperature. 1400 ml of ethyl acetate was added, and after crystallization for 4 hours, suction filtration and drying were performed to obtain the product N-hydroxyethylglucamine.
[0032] The crude N-hydroxyethyl glucosamine was heated with ethyl acetate: methanol = 1: 2.5 (volume ratio), 5% activated carbon carbon by weight of the crude product was added, stirred and refluxed for 1 hour, the a...
Embodiment 3
[0035] Add 2200ml methanol (10% water content), 1500g glucose, 510g ethanolamine, 130mg trimethylamine into a 10L high pressure reaction kettle, replace with nitrogen, add 160g carrier nickel, tighten the feeding port, pass high-purity hydrogen, and keep the pressure at 4.0~4.5MPa , slowly increase the temperature to 50 ° C, stir the reaction hydrogenation, until the reaction hydrogen absorption is not obvious (the autoclave pressure gauge will no longer drop), about 10 hours, the reaction is completed, filter while hot to remove the catalyst, and add it after cooling to room temperature 1600 ml of ethyl acetate, after crystallization for 7 hours, suction filtration, and drying to obtain the product N-hydroxyethylglucamine.
[0036] The crude N-hydroxyethyl glucamine was heated with ethyl acetate: methanol = 1: 2.3 (volume ratio), 5% activated carbon carbon by weight of the crude product was added, stirred and refluxed for 1 hour, the activated carbon was filtered off, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com