Preparation method of high molecular weight poly-morpholine-2,5-dione derivative and preparation method of the copolymer thereof
A high-molecular-weight polymorpholine technology, which is applied in the preparation of copolymers and high-molecular-weight polymorpholine-2, can solve the problems of low molecular weight, many by-products, restrictions, etc., and achieve the effect of large molecular weight and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
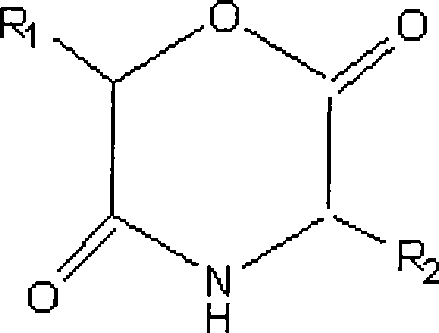
[0033] Synthesis of polymorpholine-2,5-dione derivatives of double-terminal hydroxyl groups (poly 3(S)-isopropylmorpholine-2,5-dione of double-terminal hydroxyl groups):
[0034] Install a thermometer, vacuum tube and nitrogen inlet on a 250 ml three-necked flask; mix 100 grams of 3(S)-isopropyl-morpholine-2,5-dione (IPMD) and 2 grams of 1,2-ethanedione Alcohol was added to the reaction bottle, and 0.2 grams of stannous octoate was added as a catalyst; after the reaction bottle was repeatedly evacuated and nitrogen gased three times, under the protection of nitrogen, the temperature of the oil bath was raised to 140 ° C, and the reaction was carried out for 8 hours; cooling Finally, add chloroform to dissolve, and then precipitate in ether; filter, wash, and vacuum-dry at room temperature; obtain poly 3(S)-isopropylmorpholine-2,5-dione with double-terminal hydroxyl groups.
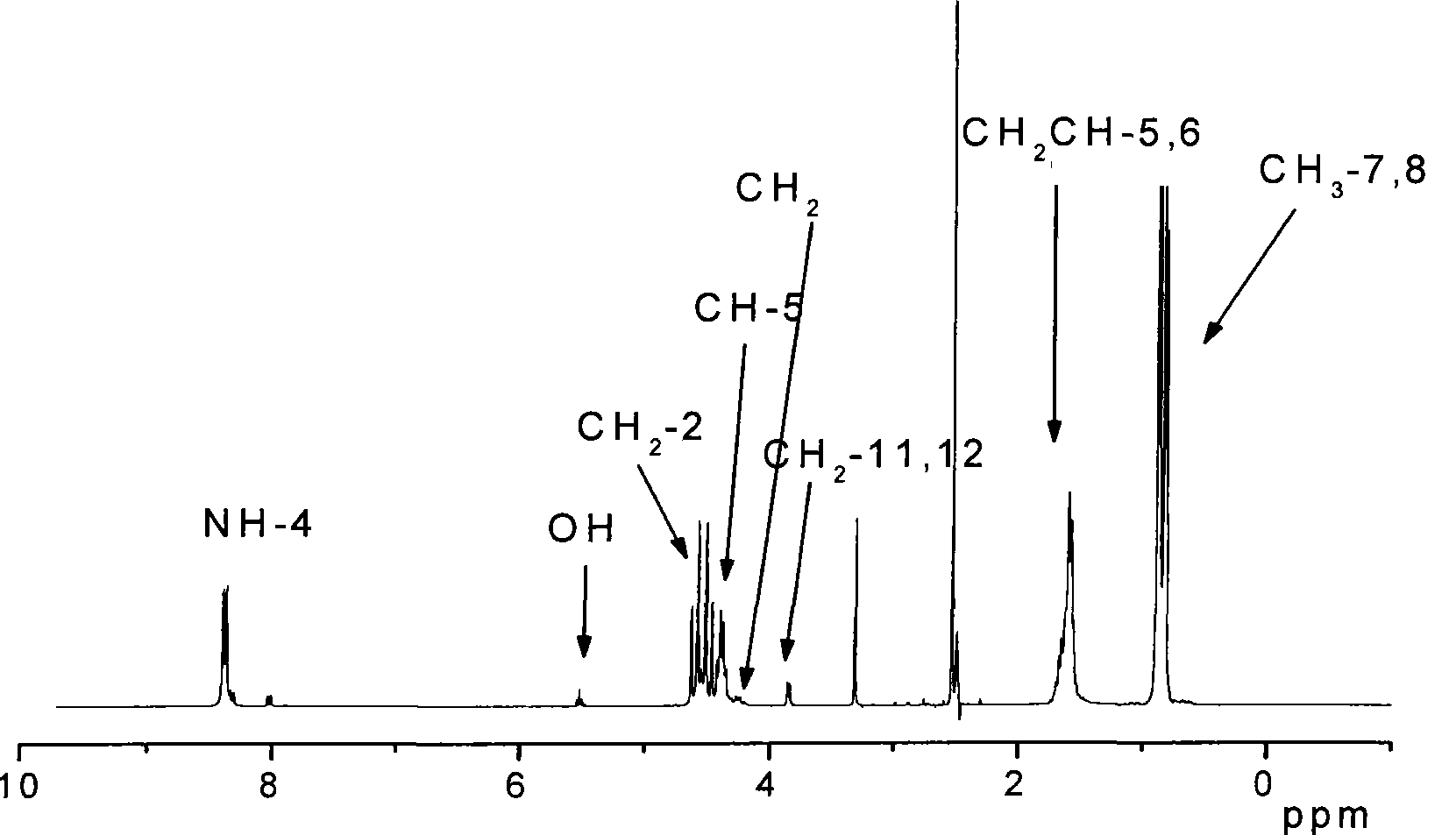
[0035] The chemical structure of the poly-3(S)-isopropylmorpholine-2,5-diketone of double-terminal hydr...
Embodiment 2
[0041] Synthesis of High Molecular Weight Polymorpholine-2,5-dione Derivatives (High Molecular Weight Poly 3(S)-isopropylmorpholine-2,5-dione)
[0042] On a 250 milliliter there-necked flask, a thermometer, a vacuum tube and a nitrogen inlet are installed; the poly 3(S)-isopropylmorpholine-2,5-dione (hydroxyl value) of the two-terminal hydroxyl prepared by 10 grams of embodiment 1 Measure and confirm that molecular weight is 2850) and 40 milliliters of chloroform, add simultaneously the stannous octoate of 0.02 gram as catalyst; Under nitrogen protection, add 736 milligrams of trimethylhexamethylene diisocyanate (TMDI), oil bath temperature is raised to 60° C. for 8 hours to make the reaction complete. The obtained polymer solution was cooled, supplemented with 40 ml of chloroform, and then precipitated in ether. Filter, wash, and dry under vacuum at room temperature. High molecular weight poly 3(S)-isopropylmorpholine-2,5-dione is obtained.
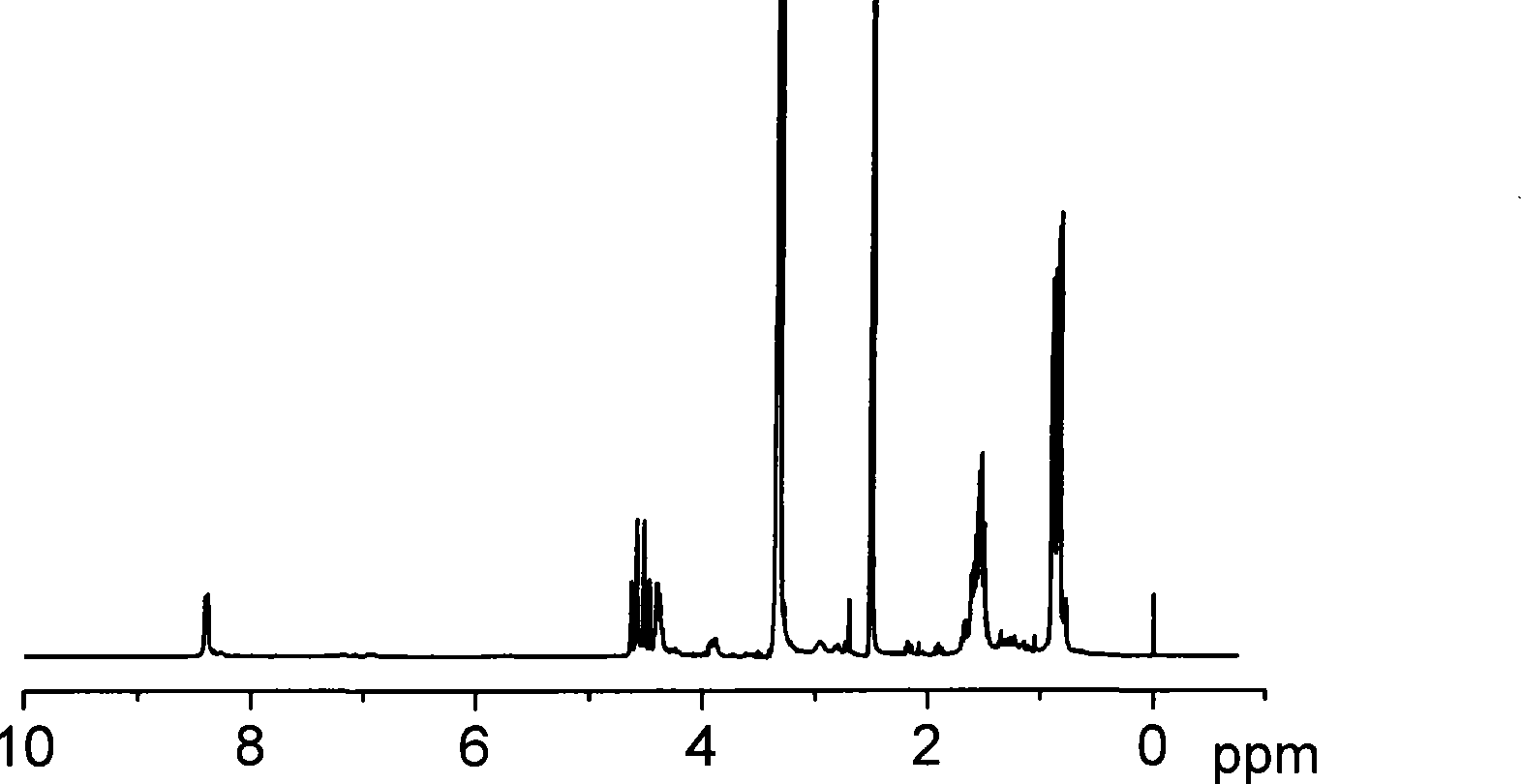
[0043] The chemical structure ...
Embodiment 3
[0050] Copolymer of polymorpholine-2,5-dione derivatives with double-terminal hydroxyl groups (poly(3(S)-isopropylmorpholine-2,5-dione-co-lactide) with double-terminal hydroxyl groups synthesis
[0051] Install a thermometer, a vacuum tube and a nitrogen inlet on a 250 ml three-necked flask; 100 grams of 3(S)-isopropyl-morpholine-2,5-dione (IPMD) and 100 grams of D,L-propane Lactide and 6 grams of 1,4-butanediol were added to the reaction flask, and 0.4 grams of stannous octoate was added as a catalyst; after the reaction flask was repeatedly evacuated and nitrogen gas flowed three times, under nitrogen protection, the temperature of the oil bath was raised to High to 140 ° C, react for 8 hours, after cooling, add chloroform to dissolve, and then precipitate in ether. Filter, wash, and dry under vacuum at room temperature. Poly(3(S)-isopropylmorpholine-2,5-dione-co-lactide) with double-terminated hydroxyl groups is obtained.
[0052] The molecular weight determined by gel p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Young's modulus | aaaaa | aaaaa |
| Breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com