Pyrrole imine vanadium olefin polymerization catalyst, preparation method and applications thereof
A pyrroleimide vanadium catalyst and a technology for olefin polymerization are applied to the pyrroleimide vanadium olefin polymerization catalyst, preparation method and application field, and can solve the problems of low molecular weight of copolymer, low copolymerization activity, poor copolymerization activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
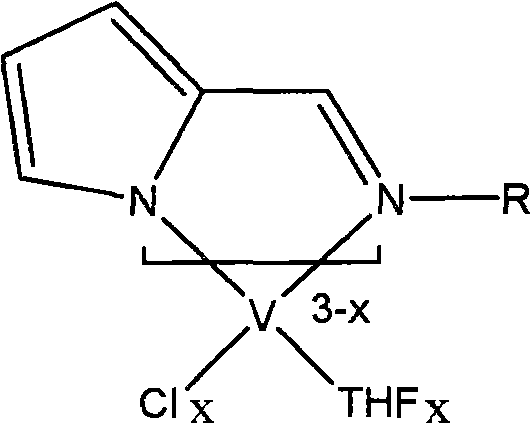
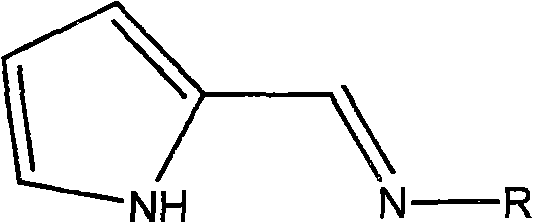
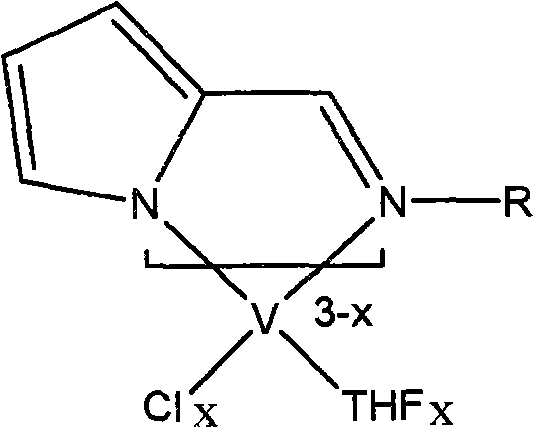
[0026] Example 1 Add 30 mg (1.25 mmol) of sodium hydride to 0.18 g (1 mmol) of Schiff base ligand and 20 ml of tetrahydrofuran at room temperature under a nitrogen atmosphere, and stir at room temperature for 4 hours. Add this anion solution dropwise to 0.37 g (1 mmol) of tetrahydrofuran complex VCl3·3THF of vanadium trichloride in 10 ml of tetrahydrofuran at room temperature, and continue to stir the reaction at room temperature for 12 h. The solvent was removed in vacuo to obtain a crude product, which was dissolved by adding 20ml of toluene and stirred for 10min. After vacuum filtration, the filtrate was concentrated to 10ml, and 20ml of n-hexane was added to precipitate a brown crystalline compound to obtain the pyrrolimide vanadium olefin polymerization catalyst 1a-1 (0.33 g, yield 75%). According to mass spectrometry, the molecular ion peak m / e is 440. Elemental analysis found values: C, 51.58%; H, 7.04%; N, 6.38%; theoretical values: C, 51.71%; H, 7.08%; N, 6.35%.
[...
Embodiment 2
[0031] Example 2 uses R as phenyl, 2,6-dimethylphenyl, 2,6-diisopropylphenyl, p-methylphenyl, p-methoxyphenyl, p-trifluoromethylphenyl Or the Schiff base ligand of pentafluorophenyl respectively replaces the Schiff base ligand in which R is cycloethyl in Example 1, and the other preparation methods are the same as catalyst 1a-1 or 1a-2 in Example 1. Correspondingly, following pyrrole imide vanadium olefin polymerization catalyst 2a-1 (75%); 2a-2 (67%); 3a-1 (87%); 3a-2 (58%); 4a-1 (73%) 4a-2 (65%); 5a-1 (76%); 5a-2 (67%); 6a-1 (77%); 6a-2 (82%); 7a-1 (65%); 7a -2 (81%); 8a-1 (79%); or 8a-2 (80%). Analysis data such as "analysis data table".
[0032] The structural formula of the pyrrole imide vanadium olefin polymerization catalyst that embodiment 1-2 obtains is as follows:
[0033]
[0034] Analysis data table:
[0035]
[0036]
Embodiment 3
[0037] Example 3 Under an ethylene atmosphere, add anhydrous toluene 50ml, 2M toluene solution of diethyl aluminum chloride 0.4ml, 0.5M toluene solution 0.12ml in a 150ml polymerization bottle successively, at 25 After stirring at -70°C for 5 minutes, add 0.2 ml of pyrrolimide vanadium olefin polymerization catalysts 1a-1 to 8a-1 and 1a-2 to 8a-2 respectively, the concentration of which is 1 μmol / ml, and polymerize under stirring for 10 minutes. Pour the reactants into 200ml of 0.5% (mass fraction) hydrochloric acid ethanol solution, filter, wash with 0.5% (mass fraction) hydrochloric acid ethanol solution, then wash with ethanol, and vacuum dry to obtain polyethylene products respectively. Ethylene homopolymerization results are shown in the table below.
[0038]
[0039]
[0040]
[0041] Note: Cat is catalyst; T is polymerization reaction temperature; W is polyethylene weight; Activity is activity; M W is the weight average molecular weight; M w / M n is the mole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com