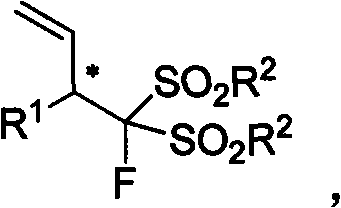
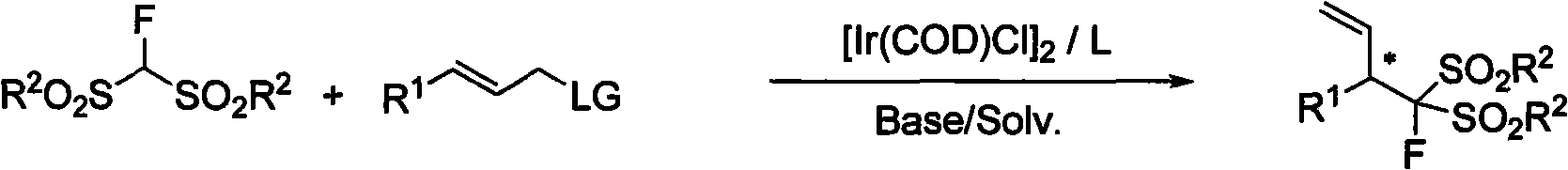3-(substituted bisulfonyl fluromethane)-1-propylene compound, synthetic method and applications thereof
A technology of disulfonyl fluoromethane and compound, which is applied in the field of allyl alkylation reaction, can solve problems such as unsolvable regioselectivity, and achieve the effects of unique physiological activity, mild reaction conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Research on the temperature and solvent of Friedel-Crafts allyl alkylation of diphenylsulfonyl fluoride with methane compounds under the catalysis of iridium complexes:
[0030]
[0031] Among them, mol refers to mole, base refers to alkali, solvent refers to solvent, and rt refers to room temperature.
[0032]
[0033]
[0034] Among them, THF is tetrahydrofuran, toluene is toluene, dioxane is dioxane, DCE is dichlorohexane, DCM is dichloromethane, Et 2 O is ether, MeCN is acetonitrile, CDCl 3 It is deuterated chloroform, DBU is 1,8-diazabicyclo[5,4,0]undec-7-ene, DABCO is triethylenediamine, urotropine is urotropine; used for serial number 1-10 The amount of alkali is 1.1 times of consumption, and sequence number 11-17 is 2.2 times of consumption.
Embodiment 2
[0035] Example 2: Research on the allylic alkylation of diphenylsulfonyl fluoromethane with different ligands under the catalysis of iridium complex:
[0036]
[0037] 1a R 3 , R 4 = Ph 1d R 3 , R 4 =Ph 1e R 5 = i Pr, Ar=Ph
[0038] 1b 3 , R 4 = 2-Naphthyl
[0039] 1c 3 , R 4 =2-MeO-Ph
[0040]
[0041] Where Ph is phenyl, Naphthyl is naphthyl, MeO is methoxy, i Pr is isopropyl.
Embodiment 3
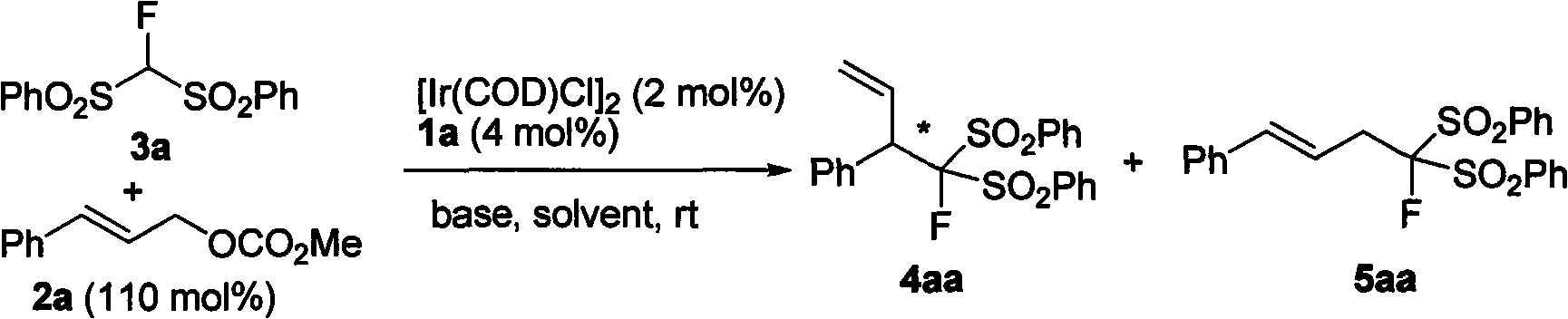
[0042] Example 3: Diphenylsulfonyl fluoromethane compounds and allyl carbonate undergo allyl alkylation under the catalysis of metal iridium complexes
[0043]
[0044] Add [Ir(COD)Cl] sequentially to a dry reaction tube 2 (0.004mmol), chiral ligand (0.008mmol), n-propylamine (0.5mL) and THF (0.5mL), reacted at 50°C for 20 minutes, then naturally cooled to room temperature and pumped dry. Diphenylsulfonylfluoromethane (0.2mmol), cesium carbonate (0.5mmol), allyl carbonate (0.22mmol), and DCM (2mL) were added to the reaction tube in turn, and the reaction was stirred at room temperature. After the reaction, the solvent was removed under reduced pressure and the residue was separated by column chromatography to obtain the product (ethyl acetate / petroleum ether=1 / 5-1 / 2, v / v).
[0045] P1: 3-(diphenylsulfonylfluoromethyl)-phenylprop-1-ene
[0046]
[0047] White solid, melting point: 127-129°C; 89% yield, 94% ee; [chiral column OD-H (0.46cmx 25cm); n-hexane / isopropanol=98 / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com