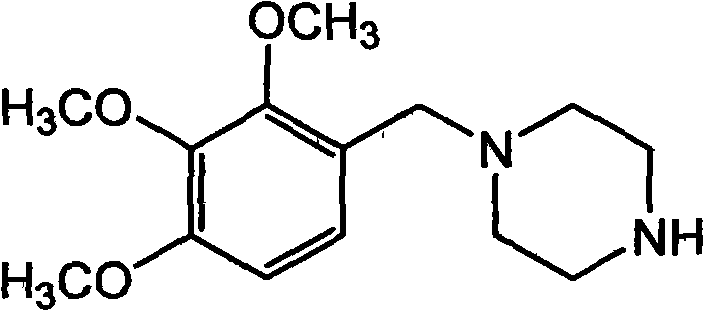
Trimetazidine and production method of hydrochloride thereof
A technology of trimetazidine and a production method, which is applied in the field of preparing anti-angina pectoris drugs, can solve the problems of high risk, high production cost, low product yield and the like, and achieve the effects of reducing production cost and enhancing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the autoclave, throw 196 grams (1.0 moles) of 2,3,4-trimethoxybenzaldehyde, 215 grams (2.5 moles) of anhydrous piperazine, 1500 milliliters of dimethoxyethyl ether and 25 grams of 5% nickel / silicon For algal earth catalyst, after purging the system with nitrogen, fill the hydrogen to 1.5MPa, raise the temperature to 75-85°C, stir and react for 6-8 hours until the reactant no longer absorbs hydrogen, cool and release the pressure, filter the reaction mixture, and pour into the filtrate Add 500 milliliters of water, adjust the pH value to 3-4 with hydrochloric acid with a concentration of 6M, separate the organic phase, wash the water phase with 150 milliliters of dichloroethane three times, and then wash the water phase with 460 grams of 50% sodium hydroxide After the solution was neutralized to pH=12, it was extracted three times with 120 ml of benzene each, the benzene extracts were combined, and the benzene was evaporated to obtain 239 g (~90%) of trimetazidine.
Embodiment 2
[0031] In the autoclave, throw 98 grams (0.5 mole) 2,3,4-trimethoxybenzaldehyde, 86 grams (1.0 mole) anhydrous piperazine, 500 milliliters of diisopropyl ether and 15 grams of 8% nickel / oxide Aluminum catalyst, after purging the system with nitrogen, fill hydrogen to 1.8MPa, heat up to 85-95°C, stir and react for 8 hours until the reactant no longer absorbs hydrogen, cool and release the pressure, filter the product, add 250 Milliliter of water, adjust the pH value to 3-4 with hydrochloric acid with a concentration of 6M, separate the organic phase, wash the aqueous phase with 100 ml of dichloroethane for 3 times, and then neutralize it with 220 grams of 50% sodium hydroxide solution. pH=12, the aqueous phase was extracted three times with 80 ml of toluene each, the toluene extracts were combined, and the toluene was evaporated to obtain 108 g (81%) of trimetazidine.
Embodiment 3
[0033] In the there-necked flask, add 400 milliliters of ethanol, 65 milliliters of 35% concentrated hydrochloric acid, under stirring, the solution of 133 grams of trimetazidine (0.5 mole) of embodiment 1 gained in 280 milliliters of ethanols is added dropwise to above-mentioned hydrochloric acid solution After the addition, stir for another 1 hour, and evaporate part of the solvent under normal pressure. After the distillate reaches 260 grams, the reaction system is cooled to 0 ° C, filtered, and the filter cake is washed with ethanol and dried to obtain 155.6 grams of trimetaz. The yield of oxazine dihydrochloride was 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com