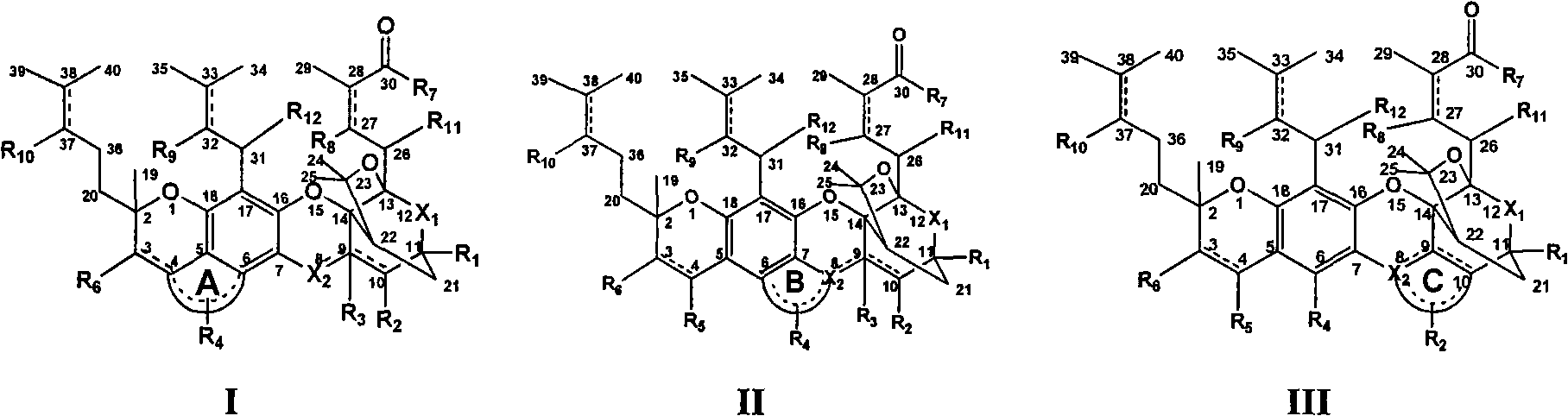
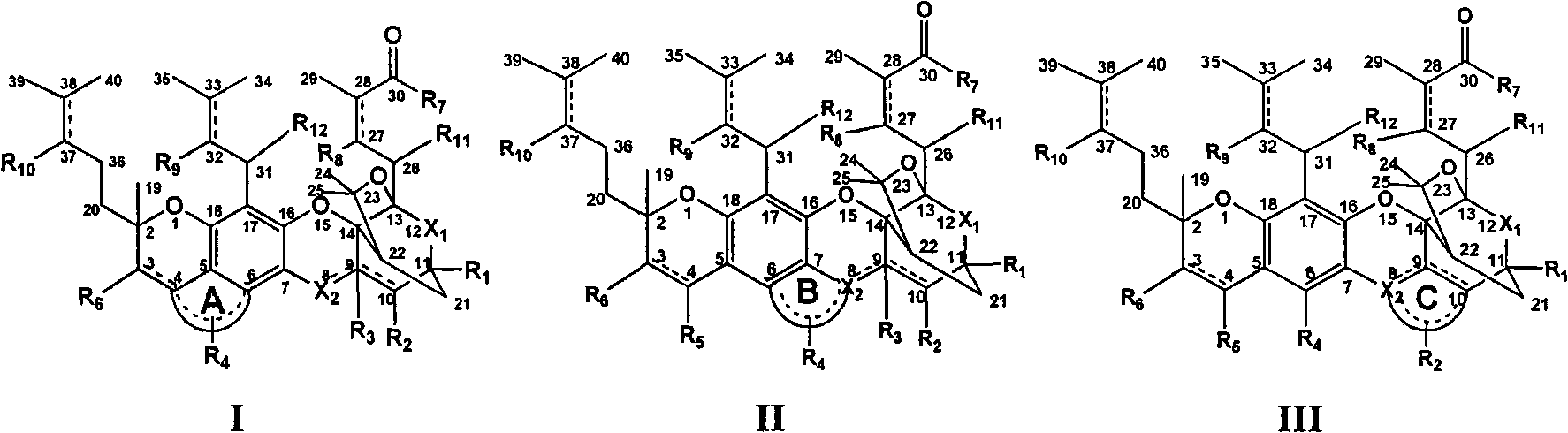
Gambogic acid cyclized analog, preparation method and application thereof
An analog, the technology of gambogic acid, which is applied to the cyclized analog of gambogic acid and the fields of its preparation and application, can solve the problems of low anti-cancer activity, high toxicity, apoptosis and the like of gambogic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] 1. Preparation of gambogic acid (see Xu Lifeng Chinese patent application 200710157223.9)
[0068] 2. Chemical synthesis and preparation
[0069] Synthesis and preparation of esters, anhydrides and amides at the 30th position of the gambogic acid ring-closure analog structure: modify the structure at the 30th position of the gambogic acid structure, modify the carboxyl group into esters and amide analogues, and use gambogic acid as the raw material , using one of the following reagents (tetrahydrofuran, 1,4-dioxane, acetonitrile, dichloromethane, N,N-dimethylformamide, N,N-dimethylacetamide, n-hexane, toluene , quinoline, etc.) as the solvent, the reaction temperature is -78 ° C to 90 ° C, using one or more catalysts, which can catalyze the formation of C-O bonds, C-S bonds, C-N bonds, C-P bonds, gambogic acid derivatives Compounds and analogs react with alcohols, thiols, acids, amines and phosphorus-containing compounds with or without substituents to produce esters, ...
Embodiment 1
[0083] Preparation of Example 1 (see Table 1 Compound 1, the same below)
[0084] Compound 1.1: In a 250ml round bottom flask, add gambogic acid 12.56g (20mmol) successively, catalytic amount 4-dimethylaminopyridine (DMAP), tetrahydrofuran (THF) 80ml, N, N dimethylformamide (DMF) 20ml, the reaction system was placed on a heat collecting type constant temperature heating magnetic stirrer, after stirring for 30 minutes, add ethanolamine 1.22g (20mmol), stir at room temperature for 8 hours, add glacial acetic acid 1.3ml, continue stirring for 8 hours, the reaction solution 40 Concentrate under reduced pressure at ℃ to distill THF, extract the aqueous phase with ethyl acetate, add anhydrous magnesium sulfate to dry, and the filtrate is recovered under reduced pressure to obtain a light yellow solid, which is separated by silica gel column chromatography to obtain compound 1.1 (see Table 1 Compound 1.1, below same). IR (KBr, cm -1 ): 3422, 2965, 2925, 2855, 1738, 1711, 1633, 1594...
Embodiment 2
[0087] Preparation of Example 2
[0088]Compound 2.1: In a 250ml round bottom flask, add 12.84g (20mmol) of methyl gambogic acid, 3.50g (24mmol) of 1,3-indanedione, 3.85g (50mmol) of ammonium acetate, and dry DMF 60ml, and react The system was placed on a collector-type constant temperature heating magnetic stirrer, the reaction system was sealed, and the reaction was stopped after stirring at 35° for 24 hours under the protection of dry nitrogen. The reaction solution was extracted by adding 150ml of ethyl acetate and 150ml of water respectively. After the ethyl acetate phase was separated, the ethyl acetate phase was extracted with 100, 100, and 50ml of water respectively. After the DMF was extracted, it was dried with anhydrous magnesium sulfate for 8 hours, the anhydrous magnesium sulfate was removed by filtration, the filtrate was mixed with 160-200 mesh silica gel, and 4.2 g of compound 2.1 was obtained by column chromatography with a yield of 27.27%. IR (KBr, cm -1 ):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com