Small peptide C with antibacterial and antitumor functions and applications thereof
A technology of anti-cancer drugs and anti-lung cancer drugs, which is applied in the field of biomedicine to achieve the effect of convenient artificial synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Peptide Design and Synthesis
[0031] 1. Extract the main international antimicrobial peptide database (http: / / aps.unmc.edu / AP / main.php; http: / / penbase.immunaqua.com / ; http: / / www.bbcm.univ.trieste.it / ; http: / / www.imtech.res.in / raghava / antibp / ) of the antibacterial peptide sequence registered in, analyze the corresponding relationship between its primary structure and activity, and design artificial short peptide sequence.
[0032] 2. Use the protein analysis database http: / / www.expasy.org / to analyze the similarity of the secondary structure of the polypeptide sequence with known activity and the designed polypeptide sequence, and correct the designed sequence in combination with other physicochemical parameter predictions.
[0033] 3. Send the peptide sequence to the biological company for synthesis or use an automatic peptide synthesizer to synthesize the complete sequence. The purity of the HPLC test should be greater than 96%, and the quality of the peptide synthe...
Embodiment 2
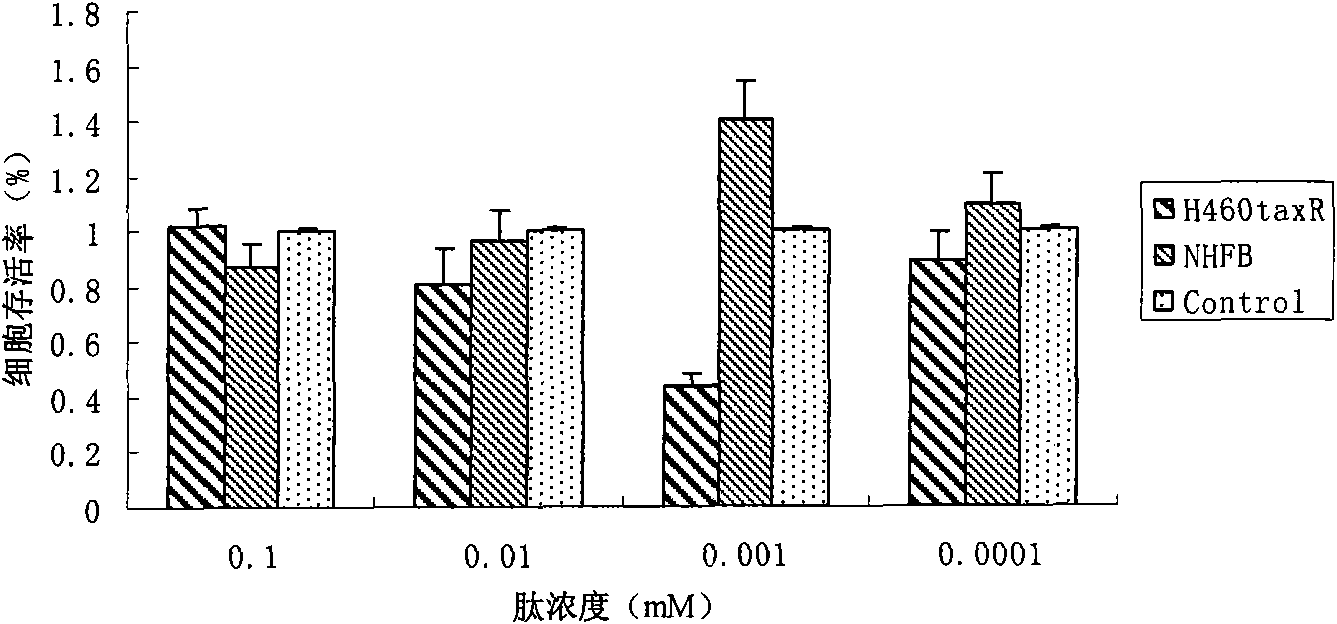
[0037] Using MTT assay to detect tumor suppressor activity of peptides in vitro
[0038] 2.1 Materials and reagents
[0039] 1) No. 2353 peptide (named small peptide C in the present invention), synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0040] 2) H460 cells (human non-small cell lung cancer line), H460 taxR Cells (human lung cancer cell line H460, resistant to paclitaxel), NHFB cells (human normal fibroblast cell line), Hela cells (human cervical cancer cell line), SGC-7901 cells (human gastric cancer cell line), Cell Bank of the Chinese Academy of Sciences / Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0041] 3) RPMI1640 medium, a product of GIBCO, USA
[0042] 4) DMEM medium, the product of HyClone Company in the United States
[0043] 5) Neonatal calf serum, a product of Hangzhou Sijiqing Company
[0044] 6) Dimethyl sulfoxide (DMSO), tetramethylazoazole (MTT) and trypsin are produc...
Embodiment 3
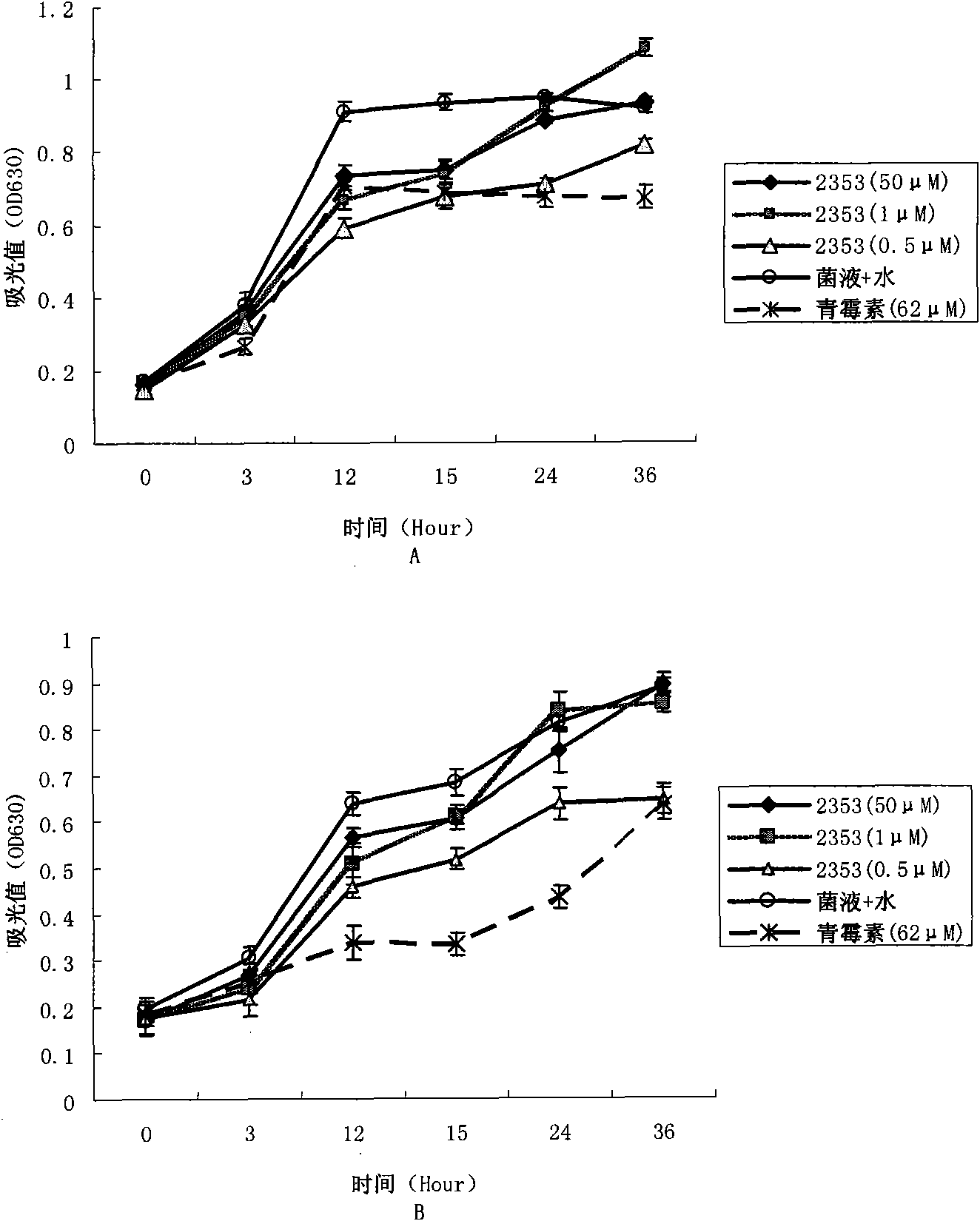
[0056] Detection of antibacterial activity of peptides by liquid growth inhibition method
[0057] 3.1 Materials and reagents
[0058] 1) Ampicillin (Amp) is a product of Solarbio Company, the disposable filter is a product of PALL Company of the United States, and other pharmaceutical reagents are of domestic analytical grade.
[0059] 2) Select Gram-positive bacteria: Bacillus subtilis (Ehrenberg) Cohn, Staphyloccocus aureus Rosenbach, Micrococcus luteus, Bacillus thuringiensis, megaspore Bacillus megaterium; Gram-negative bacteria: Escherichia coli, Vibrio, Klebsiellapneumoniae; Fungi: Fusarium solani, Fusarium oxysporum oxysporum), melon anthracnose (Colletetrichum lagenarium), apple anthracnose (Gloeosporium album) and cotton Verticillium dahliae. Provided by the strain room of the State Key Laboratory of Microbiology, Shandong University.
[0060] 3) Blocking solution: 0.4% BSA (w / v), 0.02% glacial acetic acid, filter and sterilize after preparation.
[0061] 4) PB l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com