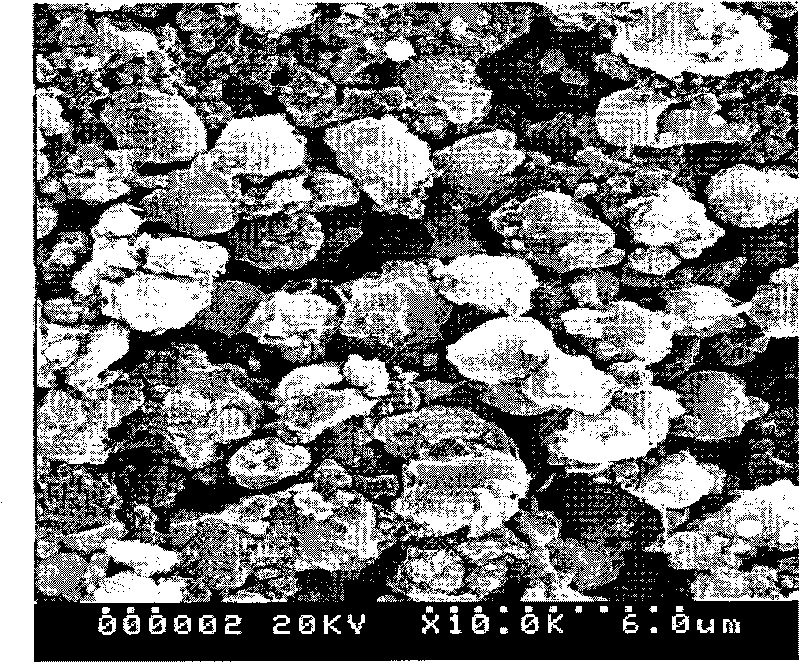
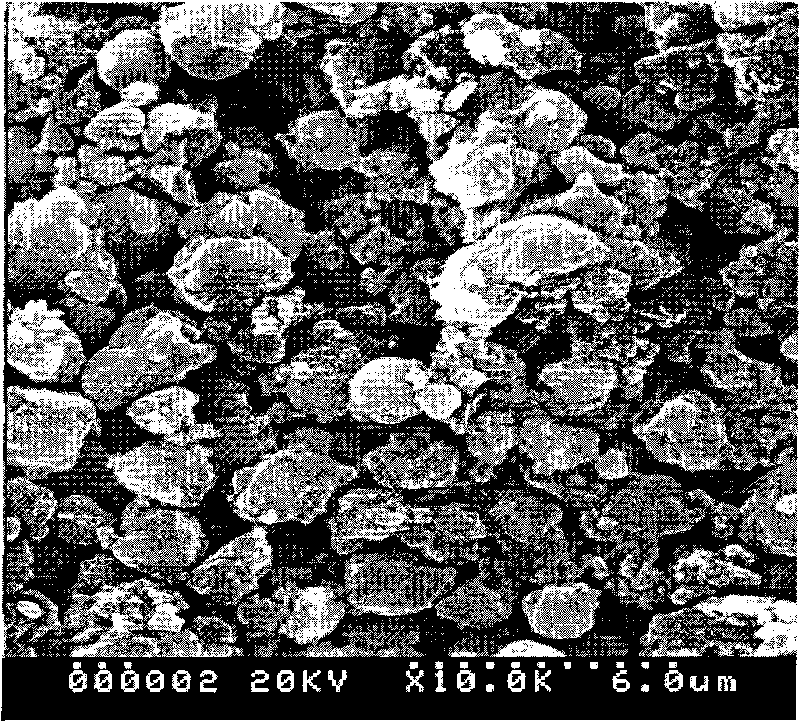
Method for preparing positive electrode material of anion-cation multi-component compound lithium battery
A multi-component composite and positive electrode material technology, applied in the direction of battery electrodes, circuits, electrical components, etc., to achieve the effect of improving the discharge platform, improving the performance of large rate performance, and good rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] After the sulfate solution of Ni, Co, Mn is mixed uniformly by Ni: Co: Mn=0.5: 0.3: 0.2 molar ratio, control pH at 11.5 with a certain amount of sodium hydroxide solution containing ammonia 5% (weight ratio) The co-precipitation reaction was realized under the conditions to generate the ternary precursor (Ni 0.5 co 0.3 mn 0.2 )(OH) 2 .
[0027] Take 0.5mol lithium carbonate Li 2 CO 3 , 0.475mol of ferric oxide Fe 2 o 3 , 0.05mol ternary precursor compound (Ni 0.5 co 0.3 mn 0.2 )(OH)2, 0.8mol concentration of 85% phosphoric acid H 3 PO 4 , 0.1mol ammonium chloride NH 4 Cl, 0.1mol tungstic acid H 2 WO 4 and 0.12mol glucose. After mixing, add water as the ball milling medium and ball mill for 8 hours, and obtain the precursor powder by spray drying. Put the precursor powder into the tube furnace under the protection of nitrogen gas, raise it to 650°C at 15°C / min, and keep the temperature for 8 hours. Then naturally cooled to room temperature and taken out t...
Embodiment 2
[0030] After the sulfate solution of Ni, Co, Mn is mixed uniformly by Ni: Co: Mn=2: 2: 1 molar ratio, control the pH at 9.5 with a certain amount of sodium hydroxide solution containing ammonia 15% (weight ratio) The co-precipitation reaction was realized under the conditions to generate the ternary precursor (Ni 0.4 co 0.4 mn 0.2 )(OH) 2 .
[0031] Take 0.5mol lithium carbonate Li 2 CO 3 , 0.475mol ferric oxide Fe 2 o 3 , 0.05mol ternary precursor (Ni 0.5 co 0.3 mn 0.2 )(OH) 2 , 0.6mol ammonium dihydrogen phosphate NH 4 h 2 PO 4 , 0.1mol ammonium chloride NH 4 Cl, 0.3mol silicic acid H 2 SiO 3 and 0.12mol glucose. After mixing, add water as the ball milling medium and ball mill for 8 hours, and obtain the precursor powder by spray drying. Put the precursor powder in the tube furnace under the protection of nitrogen gas, raise the temperature to 700°C at 15°C / min, and keep the temperature for 6 hours. Then naturally cooled to room temperature and taken out to...
Embodiment 3
[0034] After the sulfate solution of Ni, Co, Mn is mixed uniformly by Ni: Co: Mn=1: 1: 1 molar ratio, control the pH at 10 with a certain amount of sodium hydroxide solution containing ammonia 10% (weight ratio) The co-precipitation reaction was realized under the conditions to generate the ternary precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3 )(OH) 2 .
[0035] Weigh 0.5mol lithium carbonate Li 2 CO 3 , 0.475mol ferric oxide Fe 2 o 3 , 0.05mol. Ternary precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3 )(OH) 2 , 0.6mol ammonium dihydrogen phosphate NH 4 h 2 PO 4 , 0.1mol sulfur elemental S, 0.3mol boric acid H 3 BO 4 and 0.12mol glucose. After mixing, add water as the ball milling medium and ball mill for 8 hours, and obtain the precursor powder by spray drying. Put the precursor powder in the tube furnace under the protection of nitrogen gas, raise the temperature to 700°C at 15°C / min, and keep the temperature for 6 hours. Then naturally cooled to room temperature and taken out to obtain th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com