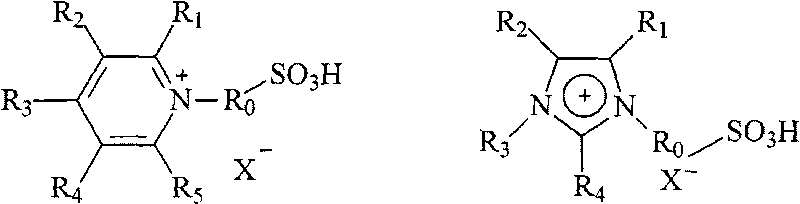Method for preparing dimer acid and dimer acid methyl ester
A dimer acid methyl ester and dimer acid technology, which is applied in the preparation of carboxylate, the preparation of carboxylate, chemical instruments and methods, etc., can solve the problems of unreusable catalyst, reduced catalyst performance, pollution of hydrolyzate, etc., To achieve the effects of environmental friendliness in the production process, fast reaction speed and high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: In 0.5L reactor, add rapeseed oil fatty acid methyl ester (main component is methyl oleate, methyl linoleate, methyl linolenate, acid value 1.58mgKOH / g oil, iodine value 104) , 3.5wt% [CH 2 SO 3 HMIM]HSO 4 For ionic liquid, the temperature is raised rapidly to about 170°C for polymerization reaction. After the polymerization reaction is over in 8 hours, the mixed solution is left to stand, cooled, and naturally settled for 2 hours (or directly centrifuged). It is crude dimer acid methyl ester (iodine value 74), and the yield is 75.0%.
[0022] The resulting crude dimer acid methyl ester is subjected to molecular distillation or conventional vacuum distillation to remove the upper light component monomer and separate the lower layer heavy component to obtain the dimer acid methyl ester.
Embodiment 2
[0023] Embodiment 2: in 0.5L reactor, add safflower seed oil fatty acid methyl ester (main component is methyl oleate, methyl linoleate, methyl linolenate, acid value 2.38mgKOH / g oil, iodine value 98 ), 4.5wt% [(CH 2 ) 4 SO 3 HMIM]HSO 4 The ionic liquid is rapidly heated to about 200°C for polymerization reaction. The polymerization reaction is completed in 6 hours, and the mixed solution is left to stand for 2 hours (or directly centrifuged), and the lower layer catalyst is separated, and the upper layer is crude dimer acid methyl ester ( The iodine value is 71), and the yield is 78.0%.
[0024] The resulting crude dimer acid methyl ester is subjected to molecular distillation or conventional vacuum distillation to remove light component monomers and separate heavy components to obtain dimer acid methyl ester.
Embodiment 3
[0025]Embodiment 3: in 0.5L reactor, add mixed unsaturated fatty acid methyl ester (the total content of oleic acid methyl ester, linoleic acid methyl ester, linolenic acid methyl ester is 92%, acid value 0.75mgKOH / g oil), 6.0wt%[(CH 2 ) 2 SO 3 H MIM] HSO 4 Ionic liquid, and rapidly heat up to about 230°C for polymerization reaction, the polymerization reaction is over in 5 hours, let the mixed solution stand still, naturally settle for 2 hours (or directly centrifuge), separate the lower catalyst, and the upper layer is crude dimer acid methyl ester (The iodine value is 68), and the yield is 81.2%.
[0026] The resulting crude dimer acid methyl ester is subjected to molecular distillation or conventional vacuum distillation to remove light component monomers and separate heavy components to obtain dimer acid methyl ester.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com