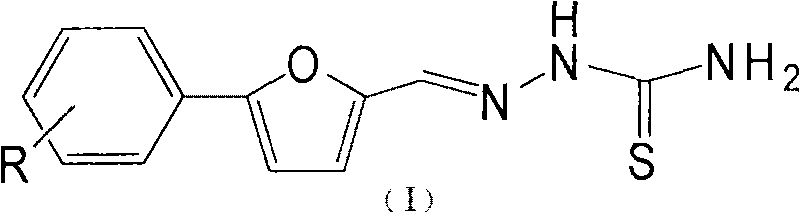
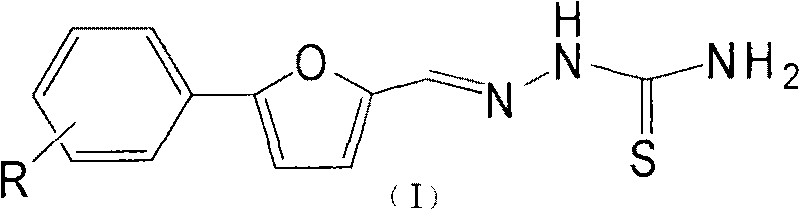
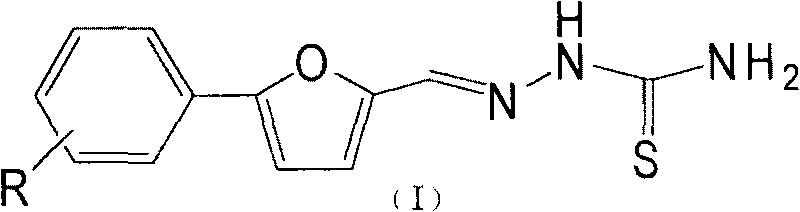
Purpose of substituent phenyl furaldehyde thiosemicarbazone compound as insect tyrosinase inhibitor
A technology of phenylfuran formaldehyde thiosemicarbazide and tyrosinase, which is applied in the directions of insecticides, animal repellents, plant growth regulators, etc., can solve the problem that L-ascorbic acid has unstable chemical properties, obvious side effects, and inhibits Problems such as poor performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the present invention takes new compound Im as example, illustrates the synthetic method of Im
[0015] Preparation of Im
[0016] Add 33g (0.1mol) of 2,4-,6-tribromoaniline, 20ml of water, and 30ml of concentrated hydrochloric acid into a 500ml four-necked flask, and stir to dissolve the solid. Then the temperature was cooled to zero in an ice bath, and 20 ml of aqueous solution of 7.0 g of sodium nitrite was slowly added dropwise. After the dropwise addition was completed, the mixture was reacted at room temperature for 40 min. Add 50ml of water, 30ml of acetone solution of 10g furfural, and 8ml of aqueous solution of 2.54g copper chloride, react at 20-25°C, add saturated sodium acetate solution dropwise until the pH reaches about 4, stop stirring, and filter with suction to obtain a yellow-brown solid. Sequentially recrystallized from petroleum ether and ethanol to obtain 9.6 g of yellow solid with a yield of 23.5%.
[0017] Add 1 g of a (2.45 mmol) o...
Embodiment 2
[0019] Embodiment 2 General formula (I) compound of the present invention, except Im, all the other compounds are known compounds, can be according to literature (Collection Czechoslov Chem Commun, 1974,39:767~771, J.Med.Chem .2002, 45, 2695-2707) described method preparation.
Embodiment 3
[0020] Example 3 Determination of the inhibitory rate of substituted phenylfuran formaldehyde thiosemicarbazones on cotton bollworm tyrosinase
[0021] a. Enzyme solution is prepared in a pre-cooled glass homogenizer in the ice-water mixture. Add one 5th instar cotton bollworm larvae, 1ml 0.1mol / L, pH=6.5 phosphate buffer, fully homogenate, and place in a centrifuge Centrifuge at a speed of 10,000×g for 15 minutes, and after suction filtration, take the supernatant in an ice bath for later use.
[0022] b. Preparation of test samples Dissolve the above compound in 1ml of DMSO, and make a 4mg / ml solution (mother solution), and dilute to different times according to the inhibition of different samples. Kojic acid and tropolone were used as control agents.
[0023] c. Determination of the compound's ability to inhibit tyrosinase activity of cotton bollworm The reaction system is 1 mL. Add 0.790mL of 0.1mol / L, pH=6.5 phosphate buffer solution, 0.05mL of enzyme solution, 10μL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com